| 规格 | 价格 | |
|---|---|---|
| 100mg | ||
| 500mg |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Nicotine and its proximate metabolite cotinine are eliminated in part by renal clearance. These compounds are filtered, secreted, and reabsorbed, and the resultant renal clearances are quite variable among individuals and are highly influenced by urine pH. In this study of 139 pairs of twins, we have estimated the genetic and environmental contributions to total renal clearance and net secretory/reabsorptive clearance of nicotine and cotinine. At uncontrolled urine pH both nicotine and cotinine undergo net reabsorption. Additive genetic factors were not important contributors to the variation in total renal clearance of nicotine but played a relatively more substantial role in accounting for the variation in total renal clearance of cotinine (43% of variance). Variations in glomerular filtration rate and the net secretory/reabsorptive clearance of nicotine and cotinine were largely influenced by nonadditive genetic influences (41.5-61% of variance). Earlier research has shown that renal secretory clearance of drugs can be highly heritable, presumably related to genetic variation in transporters. Our study suggests that the renal clearance of drugs that undergo extensive renal reabsorption can be substantially influenced by nonadditive genetic and/or shared environmental factors. Assays of metabolised cotinine are considered to be an accurate measure of exposure to cigarette smoke among pregnant women. We investigated the association and differences between the cotinine levels in maternal urine and blood, and the umbilical cord blood of three tobacco exposure groups at different stages of pregnancy. A prospective study was conducted among 398 pregnant women undergoing prenatal care in different trimesters at two medical centres and one regional hospital in central Taiwan. All 398 subjects (including 25 smokers, 191 passive smokers and 182 non-smokers) remained in the study up to the time of delivery; 384 of them delivered singleton live births. Cotinine levels were assayed in the maternal plasma and urine of the mothers at each trimester and in the cord blood of the newborns. All specimens were measured using a sensitive high-performance liquid chromatography. Cotinine concentrations in plasma and urine showed a significant dose-dependent difference among the three groups (non-smoker, passive and active smoker) and a trend that increased with gestation among the pregnant women. Significant correlations between cotinine concentrations in plasma and urine among the pregnant women in each trimester were found. In addition, the level of cotinine in umbilical cord blood was significantly correlated with that in maternal blood at term (r = 0.89, P < 0.001). A pattern of elevated cotinine concentrations in the plasma and urine of pregnant women from the beginning to the end of pregnancy was found, and this correlated significantly with the cotinine levels in the umbilical cord blood. Blood-brain barrier nicotine transfer has been well documented in view of the fact that this alkaloid is a cerebral blood flow marker. However, limited data are available that describe blood-brain barrier penetration of the major tobacco alkaloids after chronic nicotine exposure. This question needs to be addressed, given long-term nicotine exposure alters both blood-brain barrier function and morphology. In contrast to nicotine, it has been reported that cotinine (the major nicotine metabolite) does not penetrate the blood-brain barrier, yet cotinine brain distribution has been well documented after nicotine exposure. Surprisingly, therefore, the literature indirectly suggests that central nervous system cotinine distribution occurs secondarily to nicotine brain metabolism. The aims of the current report are to define blood-brain barrier transfer of nicotine and cotinine in naive and nicotine-exposed animals. Using an in situ brain perfusion model, we assessed the blood-brain barrier uptake of [3H]nicotine and [3H]cotinine in naive animals and in animals exposed chronically to S-(-)nicotine (4.5 mg/kg/day) through osmotic minipump infusion. Our data demonstrate that 1) [3H]nicotine blood-brain barrier uptake is not altered in the in situ perfusion model after chronic nicotine exposure, 2) [3H]cotinine penetrates the blood-brain barrier, and 3) similar to [3H]nicotine, [3H]cotinine blood-brain barrier transfer is not altered by chronic nicotine exposure. To our knowledge, this is the first report detailing the uptake of nicotine and cotinine after chronic nicotine exposure and quantifying the rate of blood-brain barrier penetration by cotinine. Metabolism / Metabolites Nicotine and its primary oxidative metabolites are metabolized in part by glucuronidation. Genetic variation in UGT isoenzymes that catalyze glucuronidation activity suggests that variation in glucuronidation rate is in part genetically determined. The relative contribution of genetic and environmental sources to individual differences in the rate of glucuronidation of nicotine, cotinine, and trans-3'-hydroxycotinine was estimated in a twin study of nicotine pharmacokinetics. Glucuronidation rate was defined using measures that either accounted for variability in renal clearance or assumed the same relative renal clearance of parent drug and glucuronide conjugate across individuals. The former definition resulted in highly correlated nicotine and cotinine glucuronidation measures that were substantially influenced by the combined effect of additive (heritable) and non-additive (dominant and epistatic) genetic effects. These findings suggest that genetic variation in UGT isoenzymes that act in additive and interactive ways is an important determinant of individual variability in nicotine and cotinine metabolism via glucuronidation pathways. Cotinine formation is the major pathway of nicotine metabolism in smokers, and the primary pathway of cotinine metabolism is trans-3'-hydroxylation. trans-3'-Hydroxycotinine and its glucuronide conjugate account for up to 50% of the nicotine metabolites excreted by smokers. Minor metabolites of cotinine excreted by smokers include norcotinine and cotinine N-oxide, each of which account for <5% of the nicotine dose. It has been reported that P450 2A6 is the catalyst of cotinine metabolism. However, we report here that the major product of P450 2A6-catalyzed cotinine metabolism is N-(hydroxymethyl)norcotinine, a previously unknown human metabolite of cotinine. N-(Hydroxymethyl)norcotinine was chemically synthesized, and its stability under the conditions of the enzyme reactions was confirmed. The products of P450 2A6-catalyzed [5-3H]cotinine metabolism were quantified by radioflow HPLC. The identification of N-(hydroxymethyl)norcotinine as the major metabolite was based on HPLC analysis on three unique systems and coelution with N-(hydroxymethyl)norcotinine standard. 5'-Hydroxycotinine and trans-3'-hydroxycotinine were minor products of P450 2A6-catalyzed cotinine metabolism, accounting for 14 and 8% of the total cotinine metabolites, respectively. N-(Hydroxymethyl)norcotinine was a product of cotinine metabolism by the extrahepatic P450, 2A13, but it was a minor one. The major product of P450 2A13-catalyzed cotinine metabolism was 5'-hydroxycotinine, which was formed at twice the rate of trans-3'-hydroxycotinine. The identification of all cotinine metabolites formed by both enzymes was confirmed by LC/MS/MS analysis. Kinetic parameters for cotinine metabolism were determined for P450 2A6 and P450 2A13. This work has confirmed that the major metabolite of cotinine in smokers, trans-3'-hydroxycotinine, is only a minor metabolite of P450 2A6-catalyzed cotinine metabolism. Nicotine, a major constituent of tobacco, plays a critical role in smoking addiction. In humans, nicotine is primarily metabolized to cotinine, which is further metabolized to trans-3'-hydroxycotinine. Recently, we have demonstrated that heterologously expressed human CYP2A13 is highly active in the metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), a nicotine-derived carcinogen. In the present study, CYP2A13-catalyzed NNK metabolism was found to be inhibited competitively by nicotine and N'-nitrosonornicotine (NNN), suggesting that both nicotine and NNN are also substrates of CYP2A13. We have further demonstrated that human CYP2A13 is indeed an efficient enzyme in catalyzing C-oxidation of nicotine to form cotinine, with the apparent K(m) and V(max) values of 20.2 uM and 8.7 pmol/min/pmol, respectively. CYP2A13 also catalyzes the 3'-hydroxylation of cotinine to form trans-3'-hydroxycotinine, with the apparent K(m) and V(max) values of 45.2 uM and 0.7 pmol/min/pmol, respectively. The importance of CYP2A13-catalyzed nicotine and cotinine metabolism in vivo remains to be determined. Nicotine has roles in the addiction to smoking, replacement therapy for smoking cessation, as a potential medication for several diseases such as Parkinson's disease, Alzheimer's disease, and ulcerative colitis. The absorbed nicotine is rapidly and extensively metabolized and eliminated to urine. A major pathway of nicotine metabolism is C-oxidation to cotinine, which is catalyzed by CYP2A6 in human livers. Cotinine is subsequently metabolized to trans-3'-hydroxycotinine by CYP2A6. Nicotine and cotinine are glucuronidated to N-glucuronides mainly by UGT1A4 and partly by UGT1A9. Trans-3'-hydroxycotinine is glucuronidated to O-glucuronide mainly by UGT2B7 and partly by UGT1A9. Approximately 90% of the total nicotine uptake is eliminated as these metabolites and nicotine itself. The nicotine metabolism is an important determinant of the clearance of nicotine. Recently, advances in the understanding of the interindividual variability in nicotine metabolism have been made. There are substantial data suggesting that the large interindividual differences in cotinine formation are associated with genetic polymorphisms of the CYP2A6 gene. Interethnic differences have also been observed in the cotinine formation and the allele frequencies of the CYP2A6 alleles. Since the genetic polymorphisms of the CYP2A6 gene have a major impact on nicotine clearance, its relationships with smoking behavior or the risk of lung cancer have been suggested. The metabolic pathways of the glucuronidation of nicotine, cotinine, and trans-3'-hydroxycotinine in humans would be one of the causal factors for the interindividual differences in nicotine metabolism. Cotinine has known human metabolites that include Norcotinine, trans-3'-hydroxycotinine, Cotinine N-glucuronide, and 5'-hydroxycotinine. Biological Half-Life Cotinine levels in infants are higher than in older children or adults exposed to the same reported quantity of environmental tobacco smoke. One hypothesis to explain this difference is that the urinary elimination half-life of cotinine is different between infants and older children. Urine was collected at admission, 12, 24 and 48 hr, cotinine levels were subsequently measured and then standardized by correcting for creatinine excretion. Urinary elimination half-life of cotinine was calculated in 31 infants and 23 older children. The median half-life was 28.3 hr (range 6.3-258.5 hr) in infants, and 27.14 hr (range 9.7-99.42 hr) in older children. A Mann-Whitney U test showed no significant difference in the median half-life of cotinine between the two age groups (P = 0.18). Multivariate linear regression analysis demonstrated no significant relationship between half-life of cotinine and corrected cotinine level (P = 0.24). Our results support the hypothesis that higher cotinine levels in infants is due to greater exposure, rather than slower metabolism of cotinine. The current study examined selected factors of ethnicity, menthol cigarette preference, body composition and alcohol-use history on cotinine half-life in 6 days of smoking abstinence in African American and Caucasian women. A 7-day inpatient protocol was conducted in the General Clinical Research Center, in which day 1 was ad lib smoking and days 2-7 were smoking abstinence (n = 32). Plasma cotinine was measured every 8 h throughout. Average cotinine half-life was 21.3 hr, similar to previously reported 18-20 hr. Three women exhibited >14 ng/mL cotinine after 136 hr of smoking abstinence. Host factors explaining 52.0% of variance in cotinine half-life and associated with longer half-life were being an African American menthol smoker, fewer years of alcohol use and greater lean body mass. Among menthol smokers, baseline cotinine level and cotinine half-life were not significantly different in Caucasian and African American women. |
|---|---|
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Therapeutic Category: Antidepressant. /Experimental Therapy/ /Experimental therapy/ Cotinine is the major metabolite of nicotine in humans, and the substance greatly outlasts the presence of nicotine in the body. Recently, cotinine has been shown to exert pharmacological properties of its own that include potential cognition enhancement, anti-psychotic activity, and cytoprotection. Since the metabolite is generally less potent than nicotine in vivo, we considered whether part of cotinine's efficacy could be related to a reduced ability to desensitize nicotinic receptors as compared with nicotine. Rats freely moving in their home cages were instrumented to allow ongoing measurement of mean arterial blood pressure. The ganglionic stimulant dimethylphenylpiperazinium maximally increased mean arterial blood pressure by 25 mm Hg. Slow (20 min) i.v. infusion of nicotine (0.25-1uLl) produced no change in resting mean arterial blood pressure, but the pressor response to subsequent injection of dimethylphenylpiperazinium was significantly attenuated in a dose-dependent manner by up to 51%. Pre-infusion of equivalent doses of cotinine produced the same maximal degree of inhibition of the response to dimethylphenylpiperazinium. Discrete i.v. injections of nicotine also produced a dose dependent increase in mean arterial blood pressure of up to 43 mm Hg after the highest tolerated dose. In contrast, injection of cotinine produced no significant change in mean arterial blood pressure up to 13 times the highest dose of nicotine. These results illustrate the disconnection between nicotinic receptor activation and receptor desensitization, and they suggest that cotinine's pharmacological actions are either mediated through partial desensitization, or through non-ganglionic subtypes of nicotinic receptors. |
| 分子式 |
176.21
|
|---|---|
| 分子量 |
176.21
|
| 精确质量 |
176.094
|
| CAS号 |
486-56-6
|
| PubChem CID |
854019
|
| 外观&性状 |
Light brown to brown <40°C powder,>42°C liquid
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
316.0±0.0 °C at 760 mmHg
|
| 熔点 |
40-42ºC
|
| 闪点 |
166.7±25.9 °C
|
| 蒸汽压 |
0.0±0.6 mmHg at 25°C
|
| 折射率 |
1.556
|
| LogP |
-0.23
|
| tPSA |
33.2
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
13
|
| 分子复杂度/Complexity |
205
|
| 定义原子立体中心数目 |
1
|
| SMILES |
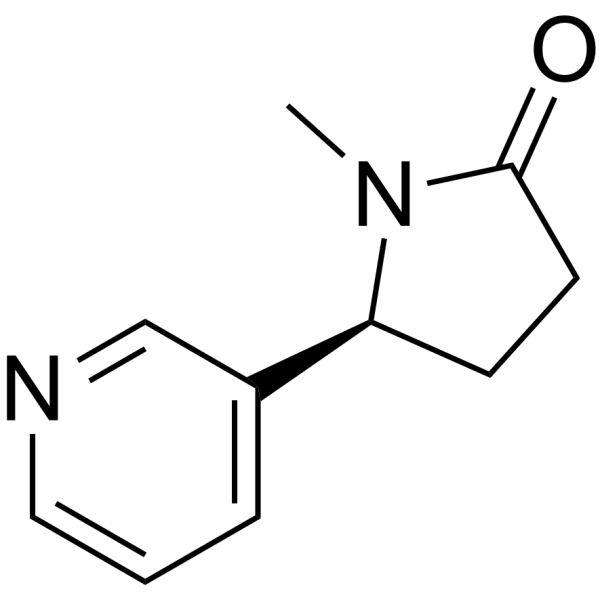
O=C1N(C)[C@H](C2=CC=CN=C2)CC1
|
| InChi Key |
UIKROCXWUNQSPJ-VIFPVBQESA-N
|
| InChi Code |
InChI=1S/C10H12N2O/c1-12-9(4-5-10(12)13)8-3-2-6-11-7-8/h2-3,6-7,9H,4-5H2,1H3/t9-/m0/s1
|
| 化学名 |
(5S)-1-methyl-5-pyridin-3-ylpyrrolidin-2-one
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
Ethanol : ~120 mg/mL (~680.97 mM)
DMSO : ~65 mg/mL (~368.86 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.17 mg/mL (12.31 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 21.7 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.17 mg/mL (12.31 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 21.7 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.17 mg/mL (12.31 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.6750 mL | 28.3752 mL | 56.7505 mL | |
| 5 mM | 1.1350 mL | 5.6750 mL | 11.3501 mL | |
| 10 mM | 0.5675 mL | 2.8375 mL | 5.6750 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。