| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| Other Sizes |
| 靶点 |
ALK (IC50 = 20 nM); c-Met (IC50 = 8 nM)
The core targets of Crizotinib (Xalkori; PF02341066) are anaplastic lymphoma kinase (ALK) and mesenchymal-epithelial transition factor (c-MET), with high selectivity for both. Specific IC50/Ki values: - c-MET (recombinant human kinase): IC50 = 11 nM [3] - ALK (recombinant human kinase): IC50 = 24 nM [3] - c-MET (cellular activity, H441 cells): IC50 = 130 nM [1] - ALK (cellular activity, Karpas 299 cells): IC50 = 60 nM [2] - ROS1 (off-target, low activity): IC50 = 170 nM [3] No significant inhibition (IC50 > 1000 nM) against non-target kinases (e.g., EGFR, VEGFR2, PDGFRα) [3] |
|---|---|
| 体外研究 (In Vitro) |
PF-2341066 在 mIMCD3 小鼠或 MDCK 犬上皮细胞中表现出类似的抗 c-Met 磷酸化功效,IC50 分别为 5 nM 和 20 nM。与 NIH3T3 细胞相比,PF-2341066 对工程表达 c-Met ATP 结合位点突变体 V1092I 或 H1094R 或 P 环突变体 M1250T 的 NIH3T3 细胞表现出改善或相似的活性,IC50 分别为 19 nM、2 nM 和 15 nM表达野生型受体,IC50 为 13 nM。相反,与野生型受体相比,观察到 PF-2341066 针对表达 c-Met 激活环突变体 Y1230C 和 Y1235D 的细胞的效力发生显着变化,IC50 分别为 127 nM 和 92 nM。 PF-2341066 还可有效防止 NCI-H69 和 HOP92 细胞中 c-Met 的磷酸化,IC50 分别为 13 nM 和 16 nM,这些细胞分别表达内源性 c-Met 变体 R988C 和 T1010I。与 c-Met 相比,PF-2341066 对 VEGFR2 和 PDGFRβ RTK 的选择性 > 1,000 倍,对 IRK 和 Lck 的选择性 > 250 倍,对 Tie2、TrkA 和 TrkB 的选择性大约 40 至 60 倍。 PF-2341066 对 RON 和 Axl RTK 的选择性是 20 至 30 倍。相比之下,PF-2341066 对 KARPAS299 人间变性大细胞淋巴瘤 (ALCL) 细胞系表达的 ALK RTK 的核磷蛋白 (NPM)-间变性淋巴瘤激酶 (ALK) 致癌融合变体显示出近乎等效的 IC50 为 24 nM。 PF-2341066 抑制癌细胞的 c-Met 依赖性肿瘤表型和内皮细胞的血管生成表型。 PF-2341066 抑制人 GTL-16 胃癌细胞生长,IC50 为 9.7 nM。 PF-2341066 诱导 GTL-16 细胞凋亡,IC50 为 8.4 nM。 PF-2341066 抑制 HGF 刺激的人 NCI-H441 肺癌细胞迁移和侵袭,IC50 分别为 11 nM 和 6.1 nM。 PF-2341066 抑制 MDCK 细胞散射,IC50 为 16 nM。 PF-2341066 可防止 HGF 刺激的 c-Met 磷酸化、细胞存活和基质胶侵袭,IC50 分别为 11 nM、14 nM 和 35 nM。此外,PF-2341066 还可防止纤维蛋白凝胶中血清刺激的 HMVEC 分支管生成(血管形成)。 PF-2341066 还可有效抑制 Karpas299 或 SU-DHL-1 ALCL 细胞中的 NPM-ALK 磷酸化,IC50 为 24 nM。 PF-2341066 有效防止细胞增殖,这与 G(1)-S 期细胞周期停滞和诱导 ALK 阳性 ALCL 细胞凋亡相关,IC50 为 30 nM,但与 ALK 阴性淋巴瘤细胞无关。此外,PF-2341066 还可预防与原发肿瘤生长(即增殖和存活)以及转移(例如侵袭和克隆性)相关的骨肉瘤行为。激酶测定:将细胞接种在 96 孔板中补充有 10% 胎牛血清 (FBS) 的培养基中,24 小时后转移至无血清培养基 [含 0.04% 牛血清白蛋白 (BSA)]。在研究配体依赖性 RTK 磷酸化的实验中,添加相应的生长因子长达 20 分钟。将细胞与 PF-2341066 和/或适当的配体孵育指定时间后,用补充有 1 mM Na3VO4 的 HBSS 洗涤细胞一次,并从细胞中产生蛋白质裂解物。随后,使用用于包被 96 孔板的特异性捕获抗体和对磷酸化酪氨酸残基具有特异性的检测抗体,通过夹心 ELISA 方法评估所选蛋白激酶的磷酸化。抗体包被板 (a) 在蛋白质裂解物存在下于 4°C 孵育过夜; (b) 用含 1% Tween 20 的 PBS 洗涤七次; (c) 在辣根过氧化物酶缀合的抗总磷酸酪氨酸 (PY-20) 抗体 (1:500) 中孵育 30 分钟; (d)再清洗七次; (e) 在 3,3',5,5'-四甲基联苯胺过氧化物酶底物中孵育以启动比色反应,通过添加 0.09 N H2SO4 来终止该反应; (f) 使用分光光度计测量 450 nm 处的吸光度。细胞测定:将包括GTL-16胃癌细胞和T47D乳腺癌细胞的细胞(GTL-16胃癌细胞和T47D乳腺癌细胞)接种到96孔板中补充有10%胎牛血清(FBS)的培养基中并转移24 小时后转移至无血清培养基 [含 0.04% 牛血清白蛋白 (BSA)]。在研究配体依赖性 RTK 磷酸化的实验中,添加相应的生长因子长达 20 分钟。将细胞与 PF-2341066 和/或适当的配体孵育指定时间后,用补充有 1 mM Na3VO4 的 HBSS 洗涤细胞一次,并从细胞中产生蛋白质裂解物。随后,使用用于包被 96 孔板的特异性捕获抗体和对磷酸化酪氨酸残基具有特异性的检测抗体,通过夹心 ELISA 方法评估所选蛋白激酶的磷酸化。抗体包被板 (a) 在蛋白质裂解物存在下于 4 °C 孵育过夜; (b) 用含 1% Tween 20 的 PBS 洗涤七次; (c) 在辣根过氧化物酶缀合的抗总磷酸酪氨酸 (PY-20) 抗体 (1:500) 中孵育 30 分钟; (d)再清洗七次; (e) 在 3,3',5,5'-四甲基联苯胺过氧化物酶底物中孵育以启动比色反应,通过添加 0.09 N H2SO4 来终止该反应; (f) 使用分光光度计测量 450 nm 处的吸光度。
1. 对c-MET/ALK驱动肿瘤的抗增殖活性: - Crizotinib抑制c-MET过表达肺腺癌细胞:H441(IC50 = 240 nM)、EBC-1(IC50 = 180 nM)[1] - 对ALK阳性间变性大细胞淋巴瘤(ALCL)细胞:Karpas 299(IC50 = 60 nM)、SU-DHL-1(IC50 = 85 nM)[2] - 对c-MET扩增胃癌细胞(MKN-45),IC50 = 210 nM [1] 2. 信号通路抑制: - 用Crizotinib(500 nM,处理2小时)处理H441细胞后,c-MET磷酸化水平(p-c-MET)及下游AKT磷酸化(p-AKT)分别降低92%和88% [1] - 在Karpas 299细胞中,100 nM Crizotinib抑制p-ALK和下游p-STAT3,抑制率分别为90%和86% [2] - 在EBC-1细胞中,300 nM Crizotinib阻断c-MET介导的ERK1/2磷酸化(p-ERK1/2)达85% [1] 3. 诱导凋亡: - 在Karpas 299细胞中,Crizotinib(200 nM,处理48小时)使凋亡率(Annexin V阳性细胞)从对照组的3.6%升至62.3%,切割型caspase-3上调4.7倍 [2] 4. 抗血管生成活性: - 在c-MET配体(HGF)刺激的人脐静脉内皮细胞(HUVECs)中,100 nM Crizotinib使管腔形成数量较对照组减少78% [1] 5. 体外PET成像关联: - 在H441细胞中,Crizotinib(300 nM)使3'-脱氧-3'-(¹⁸F)-氟胸苷(¹⁸F-FLT,细胞增殖标志物)摄取量减少65% [4] |
| 体内研究 (In Vivo) |
在 GTL-16 模型中,PF-2341066 揭示了在 50 mg/kg/天和 75 mg/kg/天治疗组中,能够使已形成的大肿瘤 (>600 mm3) 显着消退,减少 60% 43 天给药方案的平均肿瘤体积。在另一项研究中,PF-2341066 显示出完全抑制 GTL-16 肿瘤生长超过 3 个月的能力,在 50 mg/kg/ 的 3 个月治疗方案中,12 只小鼠中只有 1 只表现出肿瘤生长显着增加。天。在 NCI-H441 NSCLC 模型中,在 38 天的 PF-2341066 给药周期中,每天 50 mg/kg 时观察到平均肿瘤体积减少 43%。在 Caki-1 RCC 模型中,在 33 天的 PF-2341066 给药周期中,观察到平均肿瘤体积减少 53%,与每天 50 mg/kg/天的每个肿瘤体积减少至少 30% 相关。 PF-2341066 还显示,在 U87MG 胶质母细胞瘤或 PC-3 前列腺癌异种移植模型中,每天 50 mg/kg 剂量时,PF-2341066 几乎完全预防已形成肿瘤的生长,在最后研究日分别抑制 97% 或 84%。相比之下,以 50 mg/kg/天口服给予 PF-2341066 不会显着抑制 MDA-MB-231 乳腺癌模型或 DLD-1 结肠癌模型中的肿瘤生长。在 GTL-16 肿瘤中,在 12.5 mg/kg/天、25 mg/kg/天和 50 mg/kg/天时观察到 CD31 阳性内皮细胞的显着剂量依赖性减少,表明 MVD 的抑制显示出剂量与抗肿瘤功效的依赖性相关性。 PF-2341066 在 GTL-16 和 U87MG 模型中均显示出人 VEGFA 和 IL-8 血浆水平的显着剂量依赖性降低。口服 PF-2341066 后,在 GTL-16 肿瘤中观察到磷酸化 c-Met、Akt、Erk、PLCλ1 和 STAT5 水平的显着抑制。对携带 Karpas299 ALCL 肿瘤异种移植物的严重联合免疫缺陷米色小鼠口服 PF-2341066 会产生剂量依赖性抗肿瘤功效,在初始化合物给药 15 天内,100 mg/kg/d 剂量下所有肿瘤完全消退。此外,在浓度或剂量水平下观察到 PF-2341066 对关键 NPM-ALK 信号传导介质(包括磷脂酶 C-gamma、信号转导器和转录激活剂 3、细胞外信号调节激酶和 Akt)的抑制作用,这与抑制作用相关NPM-ALK 磷酸化和功能。 PF-2341066 可预防与原发肿瘤生长(例如增殖和存活)以及转移(例如侵袭和克隆性)相关的骨肉瘤行为。在通过口服强饲法用 PF-2341066 治疗的裸鼠中,PF-2341066 阻止了骨肉瘤异种移植物的生长以及相关的骨质溶解和皮质外骨基质形成。用 50 mg/kg PF-2341066 处理 c-MET 扩增的 GTL-16 异种移植物可引起肿瘤消退,这与 18F-FDG 摄取缓慢减少有关,并降低葡萄糖转运蛋白 1 (GLUT-1) 的表达。
1. c-MET驱动肺癌异种移植模型(H441): - 6~8周龄雌性裸鼠口服Crizotinib(100 mg/kg,每日1次,连续21天),肿瘤体积较溶媒组减少89%;中位生存期从28天延长至63天 [1] - 给药第7天的¹⁸F-FLT PET成像显示,肿瘤放射性摄取较基线减少72% [4] 2. ALK阳性ALCL异种移植模型(Karpas 299): - SCID小鼠口服Crizotinib(75 mg/kg,每日1次,连续18天),肿瘤体积较对照组减少85%;肿瘤组织Western blot显示p-ALK水平降低91% [2] 3. c-MET扩增胃癌异种移植模型(MKN-45): - 裸鼠口服Crizotinib(100 mg/kg,每日1次,连续21天),肿瘤重量较对照组减少82% [1] 4. 耐药模型(c-Myc过表达): - 携带c-Myc过表达H441异种移植瘤的小鼠对Crizotinib(100 mg/kg)反应降低:肿瘤体积减少率从亲本模型的89%降至42% [5] |
| 酶活实验 |
在 96 孔板中,将细胞接种到补充有 10% 胎牛血清 (FBS) 的培养基中,24 小时后,将细胞转移到含有 0.04% 牛血清白蛋白 (BSA) 的无血清培养基中。在研究配体依赖性 RTK 磷酸化的实验中,添加相关生长因子长达 20 分钟。将细胞与 PF-2341066 孵育一小时和/或与适当的配体孵育指定时间后,产生蛋白质裂解物。然后再次用补充有一毫克Na3VO4的HBSS洗涤细胞。之后,通过夹心 ELISA 技术评估特定蛋白激酶的磷酸化,该技术采用对磷酸化酪氨酸残基具有特异性的检测抗体和用于包被 96 孔板的特异性捕获抗体。将蛋白质裂解物添加至抗体包被的板中并在 4°C 下孵育一晚。接下来,将板在含 1% Tween 20 的 PBS 中冲洗七次,然后在辣根过氧化物酶缀合的抗总磷酸酪氨酸 (PY-20) 抗体 (1:500) 中孵育 30 分钟。最后,将板再冲洗七次。最后,将板在 3,3',5,5'-四甲基联苯胺过氧化物酶底物中孵育以启动比色反应,通过添加 0.09 N H2SO4 停止比色反应。 (f) 使用分光光度计在 450 nm 处的吸光度。
1. c-MET激酶活性实验: - 制备反应体系:含重组人c-MET激酶结构域、Crizotinib(0.1~1000 nM)、10 μM [γ-³²P]ATP及c-MET特异性肽底物(对应Tyr1234/1235自身磷酸化位点),溶于50 mM Tris-HCl缓冲液(pH 7.5,含10 mM MgCl₂、1 mM DTT)。 - 30°C孵育60分钟,加入50%三氯乙酸(TCA)终止反应。 - 磷酸化肽通过P81磷酸纤维素滤膜捕获,用0.5% TCA洗涤3次,液体闪烁计数器测定放射性强度。 - 抑制率拟合四参数逻辑模型计算IC50(IC50 = 11 nM)[3] 2. ALK激酶活性实验: - 实验方案与c-MET激酶实验一致,使用重组人ALK激酶结构域及ALK特异性肽底物。ALK的IC50 = 24 nM [3] 3. 激酶选择性实验: - 通过放射性激酶实验检测Crizotinib(1000 nM)对130种人源激酶的抑制率,仅c-MET(抑制率98%)和ALK(抑制率96%)显示显著抑制 [3] |
| 细胞实验 |
在低密度下,将肿瘤细胞接种在含有补充有 10% FBS 的生长培养基的 96 孔板中。24 小时后,将细胞转移至含有 0.04% BSA 和 0% FBS 的无血清培养基中。每个孔中填充适当的对照或指定浓度的 PF-2341066,并将细胞孵育 24 至 72 小时。将人脐带血管内皮细胞 (HUVEC) 以每孔超过 20,000 个细胞的密度接种在含有 EGM2 培养基的 96 孔板中 5 至 6 小时后,过夜转移至无血清培养基中。第二天,每个孔都充满适当的对照或指定浓度的 PF-2341066。经过一小时的孵育期后,将 100 ng/mL 的 HGF 添加到指定的孔中。为了确定相关肿瘤细胞或 HUVEC,进行了 3-(4,5-二甲基噻唑-2-基)-2,5-二苯基四唑溴化物测定。
1. 细胞增殖实验(MTT法): - 将肿瘤细胞(H441、Karpas 299、MKN-45)以5×10³细胞/孔接种于96孔板,在含10%胎牛血清的RPMI 1640培养基中过夜孵育。 - 加入Crizotinib(0.1~1000 nM),培养72小时。 - 每孔加入10 μL MTT(5 mg/mL),孵育4小时;去除培养基,加入150 μL DMSO溶解甲臜结晶,570 nm处测吸光度。 - 计算抑制增殖50%的浓度作为IC50 [1] 2. Western blot实验: - Crizotinib(100~500 nM)处理细胞2~4小时,含蛋白酶/磷酸酶抑制剂的RIPA裂解液裂解细胞。 - BCA法测定蛋白浓度,30 μg蛋白进行10% SDS-PAGE电泳,转移至PVDF膜。 - 膜用5%脱脂牛奶封闭后,4°C过夜孵育一抗(抗p-c-MET、c-MET、p-ALK、ALK、p-AKT、p-STAT3、切割型caspase-3、GAPDH)。 - 辣根过氧化物酶(HRP)标记二抗孵育后,ECL试剂检测信号 [2] 3. 凋亡实验(Annexin V/PI染色法): - Crizotinib(200 nM)处理Karpas 299细胞24/48小时,收集细胞并用冷PBS洗涤。 - 细胞重悬于结合缓冲液,加入Annexin V-FITC和PI,避光孵育15分钟,流式细胞仪分析凋亡率 [2] 4. 管腔形成实验(抗血管生成活性): - 24孔板包被基质胶,加入HUVECs(2×10⁴细胞/孔)+ HGF(50 ng/mL)+ Crizotinib(100 nM)。 - 37°C孵育6小时,拍摄图像并计数管腔分支数,计算较仅HGF刺激组的抑制率 [1] |
| 动物实验 |
PF-2341066 is administered orally by gavage to athymic mice carrying xenografts (300-800 mm 3 ) at predetermined dose levels. Mice are given PF-2341066 at predetermined intervals, and tumors are removed with humane care. Using a liquid nitrogen-cooled cryomortar and pestle, tumors are snap frozen, ground into a paste, protein lysates are produced, and protein concentrations are measured with a BSA assay. Through the use of immunoprecipitation-immunoblotting or capture ELISA, the amount of total and phosphorylated protein is measured.
1. H441 lung cancer xenograft: - Animals: Female nude mice (6–8 weeks old), n=6/group. - Tumor induction: Subcutaneous injection of 5×10⁶ H441 cells (0.2 mL PBS/Matrigel 1:1) into right flank. - Drug formulation: Crizotinib dissolved in 0.5% methylcellulose + 0.2% Tween 80. - Administration: Oral gavage at 100 mg/kg once daily for 21 days; control receives vehicle. - Monitoring: Measure tumor volume (length×width²/2) every 2 days; record survival time; ¹⁸F-FLT PET imaging on day 7 [1] 2. Karpas 299 ALCL xenograft: - Animals: Female SCID mice (6–8 weeks old), n=6/group. - Tumor induction: Subcutaneous injection of 4×10⁶ Karpas 299 cells (0.2 mL PBS/Matrigel 1:1). - Administration: Crizotinib (75 mg/kg, oral, daily for 18 days); control receives vehicle. - Endpoint: Tumor volume/weight at sacrifice; Western blot of tumor p-ALK [2] 3. c-Myc-overexpressing H441 xenograft: - Animals: Female nude mice (6–8 weeks old), n=6/group. - Tumor induction: Subcutaneous injection of 5×10⁶ c-Myc-overexpressing H441 cells. - Administration: Crizotinib (100 mg/kg, oral, daily for 21 days); control receives vehicle. - Monitoring: Tumor volume measurement; comparison of response rate vs parental xenografts [5] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
In patients with pancreatic, colorectal, sarcoma, anaplastic large-cell lymphoma and non-small cell lung cancer (NSCLC) treated with crizotinib doses ranging from 100 mg once a day to 300 mg twice a day, the mean AUC and Cmax increased in a dose-proportional manner. A single crizotinib dose of crizotinib is absorbed with a median tmax 4 to 6 hours. In patients receiving multiple doses of crizotinib 250 mg twice daily (n=167), the mean AUC was is 2321.00 ng⋅hr/mL, the mean Cmax was 99.60 ng/mL, and the median tmax was 5.0 hours. The mean absolute bioavailability of crizotinib is 43%, ranging from 32% to 66%. High-fat meals reduce the AUC0-INF and Cmax of crizotinib by approximately 14%. Age, sex at birth, and ethnicity (Asian vs non-Asian patients) did not have a clinically significant effect on crizotinib pharmacokinetics. In patients less than 18 years old, higher body weight was associated with a lower crizotinib exposure. After administering a single 250 mg radiolabeled crizotinib dose to healthy subjects, 63% and 22% of the administered dose were recovered in feces and urine. Unchanged crizotinib represented approximately 53% and 2.3% of the administered dose in feces and urine, respectively. Following a single intravenous dose, the mean volume of distribution (Vss) of crizotinib was 1772 L. At steady-state (250 mg twice daily), crizotinib has a mean apparent clearance (CL/F) of 60 L/hr. This value is lower than the one detected after a single 250 mg oral dose (100 L/hr),, possibly due to CYP3A auto-inhibition. Metabolism / Metabolites Crizotinib is mainly metabolized in the liver by CYP3A4 and CYP3A5, and undergoes an O-dealkylation, with subsequent phase 2 conjugation. Non-metabolic elimination, such as biliary excretion, can not be excluded. PF-06260182 (with two constituent diastereomers, PF-06270079 and PF-06270080) is the only active metabolite of crizotinib that has been identified. _In vitro_ studies suggest that, compared to crizotinib, PF-06270079 and PF-06270080 are approximately 3- to 8-fold less potent against anaplastic lymphoma kinase (ALK) and 2.5- to 4-fold less potent against Hepatocyte Growth Factor Receptor (HGFR, c-Met). Biological Half-Life Following single doses of crizotinib, the plasma terminal half-life was 42 hours. 1. Oral pharmacokinetics in mice: - Male C57BL/6 mice (n=3/time point) receive Crizotinib (100 mg/kg, oral). - Plasma samples collected at 0.25–24 hours; analyzed via LC-MS/MS. - Key parameters: Cmax = 2860 ng/mL, Tmax = 1.5 hours, AUC0-24h = 18900 ng·h/mL, t1/2 = 4.8 hours, oral bioavailability = 38% [1] 2. Tissue distribution: - At 2 hours post-dosing (100 mg/kg), Crizotinib concentrations (ng/g): lung (3250), tumor (2980), liver (2650), spleen (2120), brain (185) [1] 3. Plasma protein binding: - Ultrafiltration assay shows >99% protein binding in mouse, rat, dog, and human plasma (10–1000 ng/mL concentrations) [3] 4. Metabolism: - In mouse liver microsomes, Crizotinib is metabolized via CYP3A4 to two major metabolites (M1, M2); metabolic half-life = 2.3 hours [3] |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In large early clinical trials, elevations in serum aminotransferase levels occurred in up to 57% of patients treated with standard doses of crizotinib, were greater than 5 times ULN in 6% of patients, and led to early discontinuation of therapy in 2% to 4% of patients. Serum aminotransferase elevations typically arose after 4 to 12 weeks of treatment, but usually without jaundice or alkaline phosphatase elevations. Restarting crizotinib after resolution of the aminotransferase abnormalities can be done starting with a reduced dose. Most cases of liver injury due to crizotinib have been minimally or not symptomatic, and the injury resolved within 1 to 2 months of stopping the drug (Case 1). However, cases with jaundice and symptoms during crizotinib therapy have been reported which were fatal in 0.1% of treated patients (Case 2). The severe cases of liver injury due to crizotinib typically arose within 2 to 6 weeks of starting therapy and presented with marked elevations in serum aminotransferase levels followed by jaundice, progressive hepatic dysfunction, coagulopathy, encephalopathy and death. For these reasons, routine periodic monitoring of liver tests at 2 to 4 week intervals during therapy is recommended. Likelihood score: C (probable cause of clinically apparent acute liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of crizotinib during breastfeeding. Because crizotinib is 91% bound to plasma proteins, the amount in milk is likely to be low. However, its half-life is about 42 hours and it might accumulate in the infant. The manufacturer recommends that breastfeeding be discontinued during crizotinib therapy and for 45 days after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Crizotinib is 91% bound to plasma protein. _In vitro_ studies suggest that this is not affected by drug concentration. 1. Acute toxicity in mice: - Male/female C57BL/6 mice (n=3/sex/dose) receive Crizotinib (oral, 200–600 mg/kg). No mortality at 200/400 mg/kg; 600 mg/kg causes 1/6 deaths, transient weight loss (max 12% day 3, recovered day 7) [1] 2. Subacute toxicity (28-day, mice): - Doses: 50 mg/kg, 100 mg/kg (oral, daily). - 50 mg/kg group: No changes in body weight, serum biochemistry (ALT, AST, creatinine), or hematology (WBC, platelets). - 100 mg/kg group: Mild elevation of ALT (1.4× control); no histopathological damage to liver/kidneys [1] 3. Cardiac toxicity: - No QT interval prolongation in telemetered dogs treated with Crizotinib (30 mg/kg, oral) [3] 4. Drug-drug interaction: - Co-administration with CYP3A4 inhibitor (ketoconazole) increases Crizotinib AUC0-24h by 2.8-fold in mice [3] |
| 参考文献 |
|
| 其他信息 |
Pharmacodynamics
In a phase I study, 37 patients with a variety of solid-tumor cancers refractory to therapy received 50 to 300 mg of crizotinib daily or twice daily. In this group, two patients with non-small cell lung cancer (NSCLC) exhibiting echinoderm microtubule-associated protein-like 4 (EML4)-anaplastic lymphoma kinase (ALK) mutations responded to therapy; therefore, following studies focused on patients with advanced ALK-positive disease. In this group of patients, the 6-month progression-free survival among crizotinib users was approximately 72%. When compared to ALK mutation-positive patients that did not receive crizotinib, ALK mutation-positive patients treated with crizotinib had a higher two-year overall survival rate (54% vs 36%). The use of crizotinib may lead to hepatotoxicity, interstitial lung disease (ILD), pneumonitis, QT interval prolongation, bradycardia, severe visual loss, embryo-fetal toxicity and gastrointestinal toxicity in pediatric and young adult patients with anaplastic large cell lymphoma (ALCL) or pediatric patients with inflammatory myofibroblastic tumor (IMT). 1. Therapeutic background: Crizotinib (Xalkori; PF02341066) is the first dual c-MET/ALK inhibitor approved by the FDA for ALK-positive non-small cell lung cancer (NSCLC) and ROS1-positive NSCLC, addressing unmet needs in targeted therapy [3] 2. Mechanism of action: It competitively binds to the ATP-binding pockets of c-MET and ALK, inhibiting their autophosphorylation and downstream pathways (c-MET-PI3K-AKT, ALK-JAK-STAT3). It also inhibits tumor angiogenesis via blocking c-MET-mediated endothelial cell activation [1] 3. Resistance mechanism: c-Myc overexpression confers acquired resistance to Crizotinib in c-MET-addicted cancers, via upregulating alternative survival pathways (e.g., MAPK) [5] 4. Structural feature: Crizotinib has a unique 2-aminopyridine core structure that enables high affinity for both c-MET and ALK, with minimal off-target binding [3] |
| 分子式 |
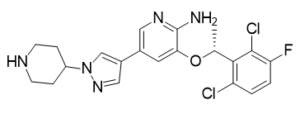
C21H22CL2FN5O
|
|---|---|
| 分子量 |
450.34
|
| 精确质量 |
449.118
|
| 元素分析 |
C, 56.01; H, 4.92; Cl, 15.74; F, 4.22; N, 15.55; O, 3.55
|
| CAS号 |
877399-52-5
|
| 相关CAS号 |
Crizotinib hydrochloride;1415560-69-8;Crizotinib-d5;1395950-84-1; 877399-53-6
|
| PubChem CID |
11626560
|
| 外观&性状 |
white to off-white solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 沸点 |
599.2±50.0 °C at 760 mmHg
|
| 闪点 |
316.2±30.1 °C
|
| 蒸汽压 |
0.0±1.7 mmHg at 25°C
|
| 折射率 |
1.673
|
| LogP |
4.73
|
| tPSA |
77.99
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
30
|
| 分子复杂度/Complexity |
558
|
| 定义原子立体中心数目 |
1
|
| SMILES |
O(C1C(N)=NC=C(C2C=NN(C3CCNCC3)C=2)C=1)[C@@H](C1C(Cl)=CC=C(F)C=1Cl)C
|
| InChi Key |
KTEIFNKAUNYNJU-GFCCVEGCSA-N
|
| InChi Code |
InChI=1S/C21H22Cl2FN5O/c1-12(19-16(22)2-3-17(24)20(19)23)30-18-8-13(9-27-21(18)25)14-10-28-29(11-14)15-4-6-26-7-5-15/h2-3,8-12,15,26H,4-7H2,1H3,(H2,25,27)/t12-/m1/s1
|
| 化学名 |
3-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-5-(1-piperidin-4-ylpyrazol-4-yl)pyridin-2-amine
|
| 别名 |
PF-2341066; PF2341066; PF02341066; PF-02341066; PF 2341066; Crizotinib; PF 02341066; US trade name: Xalkori
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1.25 mg/mL (2.78 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 12.5 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 配方 2 中的溶解度: ≥ 1 mg/mL (2.22 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 10.0 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 1 mg/mL (2.22 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 5% DMSO+30% PEG 300+dd H2O: 5 mg/mL 配方 5 中的溶解度: 20 mg/mL (44.41 mM) in 50% PEG300 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液; 需要超声助溶并加热至 40°C。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2205 mL | 11.1027 mL | 22.2054 mL | |
| 5 mM | 0.4441 mL | 2.2205 mL | 4.4411 mL | |
| 10 mM | 0.2221 mL | 1.1103 mL | 2.2205 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Targeted Treatment for ALK Positive Patients Who Have Previously Been Treated for Non-squamous Non-small Cell Lung Cancer
CTID: NCT03737994
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-11-13
Mol Cancer Ther. 2012 Jul;11(7):1557-64. |
Mol Cancer Ther. 2012 Jul;11(7):1557-64. |
 |