| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
FAK
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:在紫杉烷敏感 (SKOV3ip1) 和紫杉烷耐药 (SKOV3-TR) 细胞系中,Defactinib 显着抑制 pFAK (Tyr397) 表达。 Defactinib 和紫杉醇的组合可协同减少 SKOV3ip1、SKOV3-TR、HeyA8 和 HeyA8-MDR 细胞的增殖并增加细胞凋亡。 Defactinib 和 Y15 的组合可协同降低甲状腺癌细胞系的活力、克隆性和细胞附着。激酶测定:Defactinib 以时间和剂量依赖性方式抑制 Tyr397 位点的 FAK 磷酸化。 Defactinib 和紫杉醇的组合显着降低了增殖并增加了细胞凋亡,从而使肿瘤重量减少了 92.7% 至 97.9%。 RPPA 数据显示,Defactinib 降低了紫杉烷耐药细胞系中 AKT 和 YB-1 的水平。 FAK 抑制通过降低 YB-1 磷酸化以及随后以 AKT 依赖性方式降低 CD44 来增强紫杉烷抗性细胞的化疗敏感性。在人类卵巢癌样本中,核 FAK 表达与核 YB-1 表达增加相关 (χ2) = 37.7; P < .001)。核 FAK 和 YB-1 共表达与统计学上显着较差的中位总生存期相关(24.9 个月 vs 67.3 个月;风险比 = 2.64;95% 置信区间 = 1.38 至 5.05;P = .006)。细胞测定:用增加浓度的 Defactinib 处理卵巢癌细胞 96 小时,然后进行 MTT 测定。结果通过三次重复实验得到证实。
抑制FAK对PTX敏感性的影响[1] 我们首先测试了Defactinib/VS-6063对FAK磷酸化的体外作用。在所有细胞系中,VS-6063以剂量依赖的方式显著抑制了pFAK(Tyr397)的表达(图1A;补充图1A,可在线获得)。VS-6063在3小时内抑制了pFAK(Tyr397)的表达,48小时后表达逐渐恢复(图1B;补充图1B和2,可在线获得)。补充图3(可在线获得)代表了一个典型的实验,其中对三次实验的统计分析表明,在其他测试残基(Tyr 576/577、Tyr 925或Tyr 861)上,FAK磷酸化没有统计学上的显著变化。由于Pyk2和FAK在中心催化结构域中的相似性约为60%,我们还测试了Pyk2中的Tyr402、Tyr 579/580和Tyr 881。仅在HeyA8细胞中观察到Tyr402的磷酸化抑制,VS-6063(1µM)处理1小时后,HeyA8 MDR细胞系中的磷酸化残基没有变化。 VS-6063/Defactinib作为单一药物(0-1µM)不影响任何细胞或其紫杉烷抗性对应物的生长(补充图4,在线提供)。然而,PTX和VS-6063(1µM)的组合比单独使用任何一种药物都更有效。与单独使用PTX相比,与VS-6063联合使用PTX的细胞毒性高2.1至4.9倍(图1C;补充图1C,可在线获得)。采用Chou-Talalay法(20)用组合指数评估PTX和VS-6063的组合效果。PTX和VS-6063之间的相互作用在HeyA8和HeyA8 MDR细胞中具有协同作用(图1D)。HeyA8和HeyA8-MDR以1:1000和1:10的比例同时暴露于PTX和VS-6063剂量对细胞生长具有协同抑制作用,组合指数分别为0.953和0.705。在SKOV3ip1或SKOV3-TR细胞中,PTX和VS-6063之间没有观察到协同作用,但观察到对增殖的加性抑制作用(数据未显示)。 接下来,我们测试了基于VS-6063/Defactinib的疗法对增殖和凋亡的影响。与单独使用PTX相比,VS-6063(1µM)与PTX的组合(PTX对SKOV3和HeyA8细胞的中位抑制浓度[IC50]水平分别为8.5和6.3 nmol/L)降低了紫杉烷敏感和紫杉烷抗性细胞的增殖率(图1E;补充图1D,在线提供)。对于细胞凋亡,PTX和VS-6063联合使用的效果最大(图1F;补充图1E,可在线获得)。这些发现表明,VS-6063与PTX联合使用至少对细胞增殖和凋亡具有相加作用。与对照组(34.3%±0.3%;P<0.001)相比,PTX将HeyA8细胞中G2期细胞群的比例增加到51.0%±0.4%,与VS-6063治疗联合使用时,G2期细胞的比例在统计学上显著增加到60.8%±0.9%(与PTX相比,P<0.001)。在PTX组或联合治疗组中,HeyA8 MDR细胞均未被阻滞在G2期(P=0.05)(图1G;补充图1F,可在线获得)。 FAK抑制对下游信号的影响[1] 由于已知FAK通过β1整合素发出信号,我们首先测试了β1整合素水平是否受到Defactinib/VS-6063的影响,但没有发现统计学上的显著变化(补充图8,在线提供)。为了鉴定紫杉烷抗性细胞系中FAK下游的潜在信号通路,使用并分析了RPPA(DAVID生物信息学资源;http://david.abcc.ncifcrf.gov/).在VS-6063治疗组中,与未治疗组相比,所分析的161种蛋白质中有53种在两种耐药细胞系中显示出统计学上的显著变化(P<0.05)(图3A;补充表1,可在线获得)。我们发现AKT通路是参与最多的通路,包括pFAK(Tyr 397)、pAKT(Thr308)、GSK-3α/β(Ser21/9)、p27(T198)、p70S6K(Thr389)、PRAS40(T246)和pYB-1(Ser102)。其中,pYB-1(Ser102)是统计学上降低最显著的蛋白质(Defactinib/VS-6063治疗降低了36.6%;P<0.001)。鉴于YB-1在致癌和耐药途径中的潜在作用,我们在后续研究中重点研究了FAK和YB-1之间的潜在关系。图3B显示,PTX增加了核YB-1的表达,而PTX与VS-6063联合使用降低了其在HeyA8 MDR细胞中的表达。免疫荧光研究也揭示了类似的发现(图3C)。 FAK抑制抑制ESCC细胞的恶性进展[2] 由于FAK在几种ESCC细胞系中被过度激活,包括KYSE150、KYSE180、KYSE30、KYSE410、KYSE450、KYSE510、Colo680和KYSE70,9,我们使用MTS测定法测量了Defactinib对这些ESCC细胞株的生长抑制作用。如图1A所示,Defactinib治疗72小时 h剂量依赖性地降低了这些指示的ESCC细胞系的存活率(图1A)。我们使用Transwell试验进一步观察了defactinib在KYSE410和KYSE510中的抗侵袭或迁移能力。如图1B、C所示,defactinib剂量依赖性地抑制了指定ESCC细胞的迁移和侵袭。综上所述,这些结果表明,defactinib在ESCC细胞中具有优异的抗肿瘤作用。 接下来,FAK通过与PI3K催化亚基-p85相互作用增强PI3K/AKT通路的活性。如图2E所示,在4或24小时后,F、Defactinib剂量依赖性地破坏了FAK和PI3K p85亚基之间的相互作用 h治疗。总之,PI3K/AKT通路抑制的动力学和幅度表明,这种作用在defactinib反应中起着重要作用。 Defactinib对下游基因网络的有效抑制 为了进一步了解Defactinib治疗引起的分子效应,我们分析了用10 μM defactinib 4至24 h(图3A),并重点研究了Defactinib治疗中显著下调的转录本。如图3B-J所示,令人惊讶的是,在4或24小时后,几个靶基因在相同的功能集中大量富集,如化疗耐药性(图3B)、细胞周期(图3C)、生长因子、细胞因子和趋化因子(图3D)、恶性进展(图3E)、细胞可塑性(图3F)、热休克蛋白家族(图3G)、代谢(图3H)、癌基因(图3I)或转录因子(图3J) h defactinib治疗。对参与这些功能集的代表性基因的详细分析表明,其中许多基因在4或24小时后被defactinib持续抑制 h处理(表S1)。 |
| 体内研究 (In Vivo) |
在 PTX 敏感和 PTX 耐药模型中,Defactinib(50 mg/kg po)可增强紫杉醇对肿瘤生长的抑制作用。
VS-6063/的体内效应德法替尼[1] Defactinib/VS-6063剂量为25mg/kg,每天两次或更高,在3小时时统计学上显著抑制了pFAK(Tyr397),24小时后表达恢复(补充图5,在线提供)。因此,选择每天两次25mg/kg的Defactinib/VS-6063作为后续治疗实验的给药方案。在治疗实验中,将腹腔内携带HeyA8肿瘤的雌性裸鼠随机分为4组(每组n=10):1)每天口服两次载体,每周腹腔注射磷酸盐缓冲盐水(对照组);2)VS-6063/Defactinib25mg/kg口服,每日两次;3)每周腹腔注射PTX;4)VS-6063 25mg/kg每日口服两次,PTX每周腹腔注射一次。在HeyA8模型中,PTX单药治疗使肿瘤重量减轻了87.4%,联合治疗导致肿瘤重量减轻最大,减少了97.9%(与PTX相比P=0.05)(图2,a和B)。在SKOV3ip1模型中,与PTX相比,联合组的肿瘤重量减轻了92.7%(P<0.001)。 Defactinib对体内肿瘤恶性肿瘤的抑制有效[2] 我们进一步使用了三种异种移植模型,包括皮下肿瘤细胞接种模型(评估肿瘤生长)、腘淋巴结转移模型(评估瘤细胞的淋巴结转移能力)或肺定植模型(评估癌细胞的转移能力),以全面观察Defactinib介导的体内抗肿瘤作用。我们将KYSE410和KYSE510细胞皮下接种到BALB/c小鼠的右侧腹。当异种移植物达到约100 mm3,我们用defactinib(25mg/kg/天,口服)治疗动物约连续3周,并观察肿瘤生长。如图5A所示,治疗2周后,与对照组相比,Defactinib组的肿瘤生长明显延迟。由defactinib介导的肿瘤生长消退持续到第3周。此外,治疗3周后,Defactinib有效地阻断了指定肿瘤组织中FAK的激活(图S4)。腘淋巴结转移模型的结果显示,与对照组相比,FAK抑制显著降低了defactinib治疗组淋巴结中ESCC细胞的体积(图5B)。我们在肺定植模型中进一步评估了Defactinib对ESCC进展的影响。动物静脉注射指定的ESCC细胞。如图5C所示,defactinib治疗组肺部肿瘤巢的数量明显低于对照组。图5D的结果显示,与对照治疗相比,defactinib显著延长了携带ESCC肿瘤的动物的存活期。IHC检测显示,达法替尼显著降低了指定肿瘤中增殖生物标志物Ki-67(图5E)和血管生成生物标志物CD31(图5F)的表达。重要的是,对心脏、肝脏、脾脏和肾脏组织的组织学分析显示,对照组和Defactinib治疗组之间没有明显变化,表明Defactinib对正常组织没有产生显著的毒性作用(图6A)。Defactinib和对照治疗组的体重没有明显差异(图6B)。 |
| 酶活实验 |
以依赖时间和剂量的方式,defactinib 阻止了 Tyr397 位点的 FAK 磷酸化。德法替尼和紫杉醇一起显着减少增殖并增强细胞凋亡,导致肿瘤重量减少 92.7% 至 97.9%。 RPPA 的数据表明,在紫杉烷耐药细胞系中,德法替尼降低了 AKT 和 YB-1 水平。在紫杉烷抗性细胞中,FAK 抑制通过依赖于 AKT 的机制提高了化疗敏感性,并减少了 YB-1 磷酸化,从而减少了 CD44。核 YB-1 表达增加 (χ2) = 37.7; P <.001) 与人类卵巢癌样本中的核 FAK 表达相关。中位总生存期显着较差(24.9 个月与 67.3 个月;风险比 = 2.64;95% 置信区间 = 1.38 至 5.05;P = 0.006)与核 FAK 和 YB-1 的共表达有关。
核因子-κB转录活性的酶联免疫吸附分析[2] 根据制造商的说明,使用人核细胞因子-κB(NF-κB)p65转录因子活性检测试剂盒来评估NF-κB的活性。简而言之,将核提取物、DNA结合缓冲液、DTT和转录因子活性测定试剂加入96孔(100μl/孔)中2 h在室温下。用洗涤缓冲液洗涤四次后,向96孔板中加入NF-κB p65一抗溶液(100μl/孔)1 h在室温下。然后,依次加入辣根过氧化物酶偶联的二抗、TMB一步法底物试剂和终止溶液。NF-κB p65活性的光学密度值在450 使用微孔板阅读器读取nm。这个实验重复了五次。 微阵列分析[2] 安捷伦SurePrint G3人类基因表达v3 8 × 60 K微阵列用于评估下游基因的变化。简而言之,提取KYSE410细胞的总核糖核酸(RNA)并将其转录为互补的脱氧核糖核酸(cDNA)。然后,用花青-3-CTP标记cDNA,与微阵列杂交,用安捷伦扫描仪G2505C洗涤和扫描。使用特征提取软件提取荧光强度数据,然后使用Genespring获取原始数据。上调或下调基因的阈值是倍数变化 ≥2,p值为 ≤.05. |
| 细胞实验 |
MTT 测定是在用浓度不断增加的 defactinib 对卵巢癌细胞进行处理 96 小时后进行的。一式三份进行的实验验证了结果。
体外基因沉默[1] YB-1小干扰RNA(siRNA)1(靶序列5′-CCUGUGGCGUCGACCACA-3′)和siRNA2(靶序列5′-GUCCAGUUCAAGGCAGUA-3′),并用于抑制卵巢癌症细胞系中YB-1的表达。如前所述,使用与来自基本局部比对搜索工具(BLAST)搜索的任何已知人类mRNA不具有序列同源性的非突变siRNA作为对照。 蛋白质印迹分析[1] 如前所述制备培养细胞的裂解物。通常,30μg蛋白质通过10%十二烷基硫酸钠聚丙烯酰胺凝胶电泳分离,并转移到硝化纤维膜上。免疫印迹的更多细节见补充方法(在线提供)。 细胞活力、增殖和凋亡测定[1] 如前所述,进行了存活率[3-(4,5-二甲基噻唑-2-基)-2,5-二苯基溴化四唑(MTT)]、增殖(5-乙基il-2’-脱氧尿苷)和凋亡(膜联蛋白-V藻红蛋白[PE]/7AAD染色)测定。 细胞增殖/存活率测定[2] 使用3-(4,5-二甲基噻唑-2-基)-5-(3-羧甲氧基苯基)-2-(4-磺基苯基)-2H-四唑内盐(MTS)法评估了Defactinib对ESCC细胞的抗增殖作用。简言之,3 × 103 100个指示细胞 μl RPMI 1640培养基接种在96孔板中。24小时后,细胞被附着,并用不同剂量的Defactinib(0-25μM)处理72小时 h.然后,丢弃培养基,用MTS溶液孵育细胞1小时 h.在490℃下用分光光度法扫描板 nm,活细胞数量与甲赞产物呈正相关。 井外迁移/侵入分析[2] 使用具有含8-μm孔径的聚碳酸酯膜插入物的Transwell室系统测定细胞的迁移。所示ESCC细胞(~1× 105 细胞)在上室中接种200 μl不含FBS的RPMI 1640培养基,在下腔室中加入1 ml含有20%FBS的RPMI 1640培养基。24之后 h、 将上部腔室在甲醇中固定10分钟 用2%结晶紫溶液染色30分钟 min,用棉签去除上腔中的非迁移细胞。然后在显微镜下拍摄迁移细胞。这个实验重复了五次。 对于经孔侵入试验,上室的膜上预先涂有50 μl 2.5 mg/ml基质胶溶液。其他实验条件与迁移测定相似。 |
| 动物实验 |
Mice: The antitumor effects of defactinib are assessed by intraperitoneal injection of SKOV3ip1, SKOV3-TR, HeyA8, and HeyA8-MDR cells. Mice are randomized into four groups of ten (control, PTX alone, Defactinib alone, and PTX plus Defactinib) one week after the tumor cell injection. Three to four weeks later, treatment is started. Weekly intraperitoneal injections of PTX (2 mg/kg for SKOV3ip1 and SKOV3-TR) or 2.5 mg/kg for HeyA8 and HeyA8-MDR) are administered; twice-daily oral administration of defactinib (25 mg/kg) is recommended. HBSS was administered intraperitoneally once a week to control mice, while vehicle was given twice daily. Day 35 (SKOV3ip1 or SKOV3-TR), Day 28 (HeyA8 or HeyA8-MDR), or when any mouse appears moribund are the dates at which the mice are killed after being observed every day for side effects of the therapy. The total weight of the body, the mass and incidence of the tumor, and the quantity of tumor nodules are noted. The three methods used to treat tumors are formalin fixation, paraffin embedding, and snap freezing in a liquid nitrogen-based optimal cutting temperature (OCT) compound.
Female BALB/c nude mice aged 4 weeks old were purchased from Beijing Vital River Laboratories and maintained under standard pathogen-free conditions. All animal experiment was conducted in accordance with the protocol approved by the Institutional Review Board of Peking University Cancer Hospital & Institute. For evaluating the antitumor growth ability of Defactinib, the indicated ESCC cells (1 × 106/100 μl PBS/mouse) were subcutaneously inoculated into the right flank of each mouse (n = 5/group). When tumors reached approximately 100 mm3, animals were treated with defactinib (25 mg/kg/day, p.o.) for 3 weeks. The selected dosage and administration of defactinib were referred to previous report.17 For evaluation of FAK activity in tumor tissues, the human phospho-FAK (Tyr397) kit was used. The exact protocol was according to the manufacturer's instruction. Briefly, tumor lysates were incubated in wells of enzyme-linked immunosorbent assay plate for 2 h at 37°C. Then, lysates were discarded and detection antibody solutions were added into the appropriated well to measure the expression of phosphorylated FAK (Tyr397).[2] The antilymphatic metastasis effect of Defactinib on ESCC cells was examined using a popliteal lymph node metastasis model, which was established in mice by injecting the foot pads with the indicated ESCC cells (1 × 106/100 μl PBS/mouse, n = 5/group). Tumor and lymph node volumes were measured and calculated using the formula: length × width2 × 0.5.[2] The effect of Defactinib on ESCC metastasis was evaluated using a lung colonization model. Animals were intravenously injected with indicated ESCC cells (1 × 105/100 μl PBS/mouse, n = 5/group) and treated with defactinib. 4 weeks after injection, the mice were killed and the lungs were harvested for hematoxylin and eosin (H&E) staining.[2] For survival assay, treatments were consistent with above subcutaneous xenografted model. Survival event was recorded when tumor burden reached more than 1 cm3 in diameter or per absolute survival events. For immunohistochemical (IHC) staining evaluation of Ki-67 and CD31 expressions in tumor tissues, tumors were fixed in 10% neutral-buffered formalin for 24 h. Then, tumors were paraffin-embedded and 5 μm sections were cut. Ki-67 and CD31 antibodies (diluted at 1:500) were used for IHC staining.[2] For evaluation of toxicity of Defactinibon mice, organ tissues, including liver, heart, spleen, and kidney of mice in subcutaneous xenografted model, were collected for H&E staining.[2] |
| 药代性质 (ADME/PK) |
Pharmacokinetics [https://pubmed.ncbi.nlm.nih.gov/27025608/]
VS-6063/Defactinib was rapidly absorbed, with median Tmax observed at 2.0 h (range 0.5–4.0 h) postdose following oral doses of 200–600 mg BID. Plasma VS-6063 exposure (Cmax and AUC) increased in a less than dose proportional manner and the mean AUC0-12 and AUC0-24 values remained relatively consistent across the full dose range evaluated (Fig. 1). Doses above 400 mg BID did not result in a significant increase in VS-6063 exposure. A similar Cmax was observed in the 400 and 600 mg BID dose cohorts on both Days 1 and 15 and mean AUC values were relatively consistent across the 200–600 mg BID dose range. Clearance appeared to be increased with dose on both Days 1 and 15, which is consistent with the less than dose proportional increase in exposure. Median half-life values ranged from 2.3 to 4.3 h across all dose regimens and both PK study days. The Day 1 mean CL/F values were 45.6, 105, and 204 L/h for the 200, 400, and 600 mg doses, respectively. The Day 15 mean CL/F values were 32. 1, 70, and 123 L/h for the 200, 400, and 600 mg doses, respectively (Table 3). VS-6063 was detected in the urine of all patients and appeared consistent across all dose cohorts. The mean renal clearance (CLr) values ranged from 0.0855 to 0.179 L/h, and the percent relative to the total dose administered ranged from 0.0439 to 0.356 %. All 9 subjects had systemic concentrations of the 4 metabolites of VS-6063/Defactinib that were evaluated (M2, M3, M4, and M5). Median plasma Tmax values for all metabolites were observed at 2.0–4.0 h postdose administration for all cohorts on both PK sampling days. Based on the relative plasma Cmax and AUC0-12 values for the metabolites compared to VS-6063 values, M2 was the most abundant metabolite, followed by M4, M3, and then M5. Both the M2 and M4 exposures appeared to be >10 % of the parent exposure, while M3 and M5 had exposures that were <10 % of the parent exposure. In the urine, the M2 metabolite was the most abundant and was excreted in amounts greater than the parent compound. |
| 毒性/毒理 (Toxicokinetics/TK) |
Safety and tolerability [https://pubmed.ncbi.nlm.nih.gov/27025608/]
Treatment-related adverse events (AEs) occurring in at least two subjects are summarized in Table 2. The most commonly reported AEs overall were unconjugated hyperbilirubinemia (7 patients, 78 %), fatigue (6 patients, 67 %), decreased appetite (4 patients, 44 %), and diarrhea (3 patients, 33 %). Only one patient in the 200 mg dose cohort experienced a Grade 3 AE of unconjugated hyperbilirubinemia. All other toxicities were manageable and were predominantly mild in intensity (Grade 1 or Grade 2) in severity. There were no AEs leading to death or SAEs, and no AEs leading to early study withdrawal. No DLTs were reported in any dose cohort. Hyperbilirubinemia was asymptomatic and its onset typically occurred within the first 2 weeks of initiating treatment. Patients with Grade 1 or 2 unconjugated hyperbilirubinemia were able to continue dosing, although bilirubin levels tended to fluctuate during treatment. Hyperbilirubinemia was reported across all dose cohorts, for 3 (100 %) patients in the 200 mg dose cohort, 2 (67 %) patients in the 400 mg dose cohort, and 2 (67 %) patients in the 600 mg dose cohort. One event of hyperbilirubinemia (200 mg cohort) was Grade 3 in severity. This patient had Grade 1–2 hyperbilirubinemia starting on Day 7; the Grade 3 event began on Day 42 and resolved 6 days after onset following interruption of study drug. All reports of hyperbilirubinemia were considered to be related to Defactinib. None of these subjects had concurrent increases in ALT or AST above ULN. The most common events of gastrointestinal disorders were diarrhea reported in 3 (33 %) subjects and nausea reported in 2 (22 %). Diarrhea was reported in 1 (33 %) subject in the 400 mg dose cohort and 2 (67 %) subjects in the 600 mg dose cohort. Nausea was reported in 1 (33 %) subject in the 200 mg dose cohort, and 1 (33 %) subject in the 600 mg dose cohort. Both reports of nausea were mild in severity as were 2 of the 3 reports of diarrhea; 1 subject in the 600 mg group experienced diarrhea of moderate intensity. No clinically meaningful changes in any ECG parameter were observed for any dose cohort and no subject had a QTc interval ≥500 ms or QTc increase from baseline >30 ms. |
| 参考文献 |
|
| 其他信息 |
Defactinib Hydrochloride is the hydrochloride salt form of defactinib, an orally bioavailable, small-molecule focal adhesion kinase (FAK) inhibitor with potential antiangiogenic and antineoplastic activities. Defactinib inhibits FAK, which may prevent the integrin-mediated activation of several downstream signal transduction pathways, including those involving RAS/MEK/ERK and PI3K/Akt, thus inhibiting tumor cell migration, proliferation, survival, and tumor angiogenesis. The tyrosine kinase FAK, a signal transducer for integrins, is normally activated by binding to integrins in the extracellular matrix (ECM) but may be upregulated and constitutively activated in various tumor cell types.
Defactinib has been investigated for the treatment of Malignant Pleural Mesothelioma. Defactinib is an orally bioavailable, small-molecule focal adhesion kinase (FAK) inhibitor with potential antiangiogenic and antineoplastic activities. Defactinib inhibits FAK, which may prevent the integrin-mediated activation of several downstream signal transduction pathways, including those involving RAS/MEK/ERK and PI3K/Akt, thus inhibiting tumor cell migration, proliferation, survival, and tumor angiogenesis. The tyrosine kinase FAK, a signal transducer for integrins, is normally activated by binding to integrins in the extracellular matrix (ECM) but may be upregulated and constitutively activated in various tumor cell types. See also: Defactinib Hydrochloride (annotation moved to). Background We previously found focal adhesion kinase (FAK) inhibition sensitizes ovarian cancer to taxanes; however, the mechanisms are not well understood. Methods We characterized the biologic response of taxane-resistant and taxane-sensitive ovarian cancer models to a novel FAK inhibitor (VS-6063). We used reverse-phase protein arrays (RPPA) to identify novel downstream targets in taxane-resistant cell lines. Furthermore, we correlated clinical and pathological data with nuclear and cytoplasmic expression of FAK and YB-1 in 105 ovarian cancer samples. Statistical tests were two-sided, and P values were calculated with Student t test or Fisher exact test. Results We found that VS-6063 inhibited FAK phosphorylation at the Tyr397 site in a time- and dose-dependent manner. The combination of VS-6063 and paclitaxel markedly decreased proliferation and increased apoptosis, which resulted in 92.7% to 97.9% reductions in tumor weight. RPPA data showed that VS-6063 reduced levels of AKT and YB-1 in taxane-resistant cell lines. FAK inhibition enhanced chemosensitivity in taxane-resistant cells by decreasing YB-1 phosphorylation and subsequently CD44 in an AKT-dependent manner. In human ovarian cancer samples, nuclear FAK expression was associated with increased nuclear YB-1 expression (χ 2 = 37.7; P < .001). Coexpression of nuclear FAK and YB-1 was associated with statistically significantly worse median overall survival (24.9 vs 67.3 months; hazard ratio = 2.64; 95% confidence interval = 1.38 to 5.05; P = .006). Conclusions We have identified a novel pathway whereby FAK inhibition with VS-6063 overcomes YB-1–mediated paclitaxel resistance by an AKT-dependent pathway. These findings have implications for clinical trials aimed at targeting FAK.[1] The clinical therapeutic efficacy toward esophageal squamous cell carcinoma (ESCC) is undesirable, due to the lack of targeted agents. Focal adhesion kinase (FAK), a nonreceptor tyrosine kinase involved in multiple fields of tumorigenesis, recently has been indicated as a promising therapeutic target in ESCC treatment. Here, we revealed that defactinib, a specific FAK inhibitor, effectively suppressed the malignancy of ESCC cells. Mechanistically, defactinib dose and time-dependently induced the dissociation of phosphoinositide-3-kinase (PI3K) from FAK, resultantly led to blockade of protein kinase B (AKT) signaling, and the expression of several oncogenes, such as SOX2, MYC, EGFR, MET, MDM2, or TGFBR2, identified by microarray and real-time polymerase chain reaction assay. Specifically, this FAK inhibition-mediated suppression of PI3K/AKT signaling and downstream ESCC specific biomarkers was maintained to 24 h in in vitro experiments to guarantee the treatment durability and efficacy. Importantly, defactinib suppressed tumor growth, metastatic ability, and increased overall survival of xenografted animals without producing significantly systematic toxicity. Our data suggest that FAK inhibition provides an excellent targeted therapy toward ESCC by effectively inhibiting PI3K/AKT pathway and downstream molecular network.[2] |
| 分子式 |
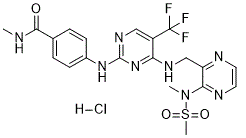
C20H22CLF3N8O3S
|
|---|---|
| 分子量 |
546.95
|
| 精确质量 |
546.118
|
| 元素分析 |
C, 43.92; H, 4.05; Cl, 6.48; F, 10.42; N, 20.49; O, 8.78; S, 5.86
|
| CAS号 |
1073160-26-5
|
| 相关CAS号 |
Defactinib;1073154-85-4; 1073160-26-5; 1345713-71-4
|
| PubChem CID |
25117347
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
4.165
|
| tPSA |
153.71
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
13
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
36
|
| 分子复杂度/Complexity |
804
|
| 定义原子立体中心数目 |
0
|
| SMILES |
Cl[H].S(C([H])([H])[H])(N(C([H])([H])[H])C1C(C([H])([H])N([H])C2C(C(F)(F)F)=C([H])N=C(N([H])C3C([H])=C([H])C(C(N([H])C([H])([H])[H])=O)=C([H])C=3[H])N=2)=NC([H])=C([H])N=1)(=O)=O
|
| InChi Key |
RCHQNUQAHJNRBY-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C20H21F3N8O3S.ClH/c1-24-18(32)12-4-6-13(7-5-12)29-19-28-10-14(20(21,22)23)16(30-19)27-11-15-17(26-9-8-25-15)31(2)35(3,33)34;/h4-10H,11H2,1-3H3,(H,24,32)(H2,27,28,29,30);1H
|
| 化学名 |
N-methyl-4-[[4-[[3-[methyl(methylsulfonyl)amino]pyrazin-2-yl]methylamino]-5-(trifluoromethyl)pyrimidin-2-yl]amino]benzamide;hydrochloride
|
| 别名 |
VS-6063 HCl; PF-04554878 HCl; Defactinib hydrochloride; Defactinib HCl; VS6063 HCl; VS 6063 HCl; VS-6063 HCl; PF04554878 HCl; PF 04554878 HCl; PF4554878 HCl; PF-4554878 HCl; PF4554878 HCl; 1073160-26-5; Defactinib hydrochloride [USAN]; UNII-L2S469LM49; VS-6063 HYDROCHLORIDE; L2S469LM49; Defactinib hydrochloride (USAN); DEFACTINIB HYDROCHLORIDE [WHO-DD];
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 0.67 mg/mL (1.22 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 6.7 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 0.67 mg/mL (1.22 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 6.7mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 0.67 mg/mL (1.22 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 5% DMSO+50% PEG 300+5% Tween 80+ddH2O: 5mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8283 mL | 9.1416 mL | 18.2832 mL | |
| 5 mM | 0.3657 mL | 1.8283 mL | 3.6566 mL | |
| 10 mM | 0.1828 mL | 0.9142 mL | 1.8283 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04439331 | Active Recruiting |
Drug: Defactinib Hydrochloride | Refractory Lymphoma Advanced Lymphoma |
National Cancer Institute (NCI) |
August 12, 2015 | Phase 2 |
| NCT04720417 | Active Recruiting |
Drug: Defactinib Hydrochloride Procedure: Biopsy |
Metastatic Uveal Melanoma | Thomas Jefferson University | January 26, 2021 | Phase 2 |
| NCT02465060 | Active Recruiting |
Drug: Defactinib Drug: Defactinib Hydrochloride |
Lymphoma Melanoma |
National Cancer Institute (NCI) |
August 17, 2015 | Phase 2 |
 In vitro biological effects of VS-6063 on taxane-sensitive and taxane-resistant cell lines.J Natl Cancer Inst.2013 Oct 2;105(19):1485-95. |
|---|
 In vivo effects of VS-6063 combined with paclitaxel (PTX).J Natl Cancer Inst.2013 Oct 2;105(19):1485-95. |
 VS-6063 restores YB-1–mediated paclitaxel (PTX) resistance.J Natl Cancer Inst.2013 Oct 2;105(19):1485-95. |
 VS-6063 downregulated YB-1 phosphorylation and nuclear translocation in taxane-resistant cells by an AKT-dependent pathway.J Natl Cancer Inst.2013 Oct 2;105(19):1485-95. |
|---|
 FAK inhibition or silencing of YB-1 downregulates the expression of CD44.J Natl Cancer Inst.2013 Oct 2;105(19):1485-95. |
Impact of pFAK (Tyr 397) and pYB-1 (Ser 102) expressions on patient survival.J Natl Cancer Inst.2013 Oct 2;105(19):1485-95. |