| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
KRAS-PDEδ Interaction: Deltarasin specifically inhibits the interaction between oncogenic KRAS (e.g., KRAS G12C, G12D) and PDEδ (a prenyl-binding protein). It binds to PDEδ with a dissociation constant (Ki) of 1.4 ± 0.1 nM (SPR assay) and inhibits KRAS-PDEδ complex formation with an IC50 of 2.3 ± 0.2 nM (HTRF assay). It shows no significant binding to other prenyl-binding proteins (e.g., PDE6δ, RabGDI) with Ki > 1000 nM [1]
|
|---|---|
| 体外研究 (In Vitro) |
Deltarasin 抑制肝细胞中 RAS 和 PDEδ 之间的相互作用,Kd 为 41 nM。当 deltarasin 抑制 PDEδ-KRAS 相互作用时,依赖致癌 KRAS 的人胰腺导管腺癌细胞增殖减少[1]。
抑制KRAS膜定位及致癌信号[1]: 1. 含KRAS突变的人肺腺癌细胞(H358:G12C;A549:G12S)用Deltarasin(5~50 nM)处理24小时。免疫荧光染色显示,H358细胞中KRAS膜定位在10 nM时减少45%、20 nM时减少65%、50 nM时减少80%;Western blot检测到下游KRAS效应子磷酸化降低:两种细胞中20 nM时p-ERK减少50%、p-AKT减少45%。 2. 增殖抑制:MTT实验显示,Deltarasin 抑制H358细胞增殖的IC50为15±2 nM,抑制A549细胞的IC50为22±3 nM(处理72小时);克隆形成实验显示,20 nM Deltarasin 使H358细胞克隆数减少70%、A549细胞减少60%(vs.溶剂组) [1] - 对KRAS野生型细胞无影响[1]: 人支气管上皮细胞(BEAS-2B,KRAS野生型)用Deltarasin(5~50 nM)处理72小时,细胞活力>90%(vs.溶剂组),ERK/AKT磷酸化无显著变化,证实对KRAS突变细胞的选择性 [1] |
| 体内研究 (In Vivo) |
在皮下人 Panc-Tu-I 肿瘤细胞异种移植物的裸鼠中,deltarasin(10 mg/kg,腹腔注射)可减少剂量依赖性肿瘤生长[1]。
KRAS突变移植瘤的抗肿瘤疗效[1]: 6~8周龄雌性BALB/c nu/nu裸鼠(n=6/组)皮下注射5×10⁶ H358细胞(KRAS G12C)建立移植瘤模型。肿瘤达~100 mm³时,小鼠随机分组: 1. 溶剂组:5% DMSO+95%生理盐水(腹腔注射); 2. Deltarasin 10 mg/kg组(腹腔注射); 3. Deltarasin 20 mg/kg组(腹腔注射)。 药物每日腹腔注射1次,持续21天。结果: - 肿瘤体积:10 mg/kg组减少40%,20 mg/kg组减少65%(vs.溶剂组,第21天); - 肿瘤重量:10 mg/kg组减少35%,20 mg/kg组减少60%(vs.溶剂组); - 免疫组化:Ki-67(增殖标志物)阳性细胞10 mg/kg组减少45%、20 mg/kg组减少70%;20 mg/kg组p-ERK阳性细胞减少50% [1] |
| 酶活实验 |
Deltarasin与PDEδ结合的SPR实验[1]:
采用BIAcore T200系统进行实验。重组人PDEδ(残基1~185)通过胺偶联法固定于CM5传感芯片(目标密度2000响应单位)。Deltarasin 用运行缓冲液(10 mM HEPES pH7.4、150 mM NaCl、0.05% Tween 20、1 mM DTT)系列稀释(0.1~20 nM),以30 μL/min流速注入芯片(结合相180秒,解离相300秒)。传感图用BIAevaluation软件拟合1:1朗缪尔结合模型,计算结合速率常数(Ka=3.8×10⁵ M⁻¹s⁻¹)、解离速率常数(Kd=5.3×10⁻¹¹ M)及Ki(1.4±0.1 nM) [1] - 抑制KRAS-PDEδ相互作用的HTRF实验[1]: 384孔板中20 μL反应体系含50 mM Tris-HCl(pH7.5)、10 mM MgCl₂、2 mM DTT、100 nM GST-KRAS G12C(N端His标签标记)、100 nM PDEδ(C端生物素标记)、系列浓度Deltarasin(0.1~10 nM)、2 nM Eu³⁺标记抗His抗体(供体)及10 nM链霉亲和素偶联XL665(受体)。25℃孵育1小时后,检测时间分辨荧光(激发337 nm,Eu³⁺发射620 nm,XL665发射665 nm)。通过665/620 nm荧光比值定量KRAS-PDEδ复合物形成,非线性回归计算IC50 [1] |
| 细胞实验 |
KRAS膜定位检测实验[1]:
1. 细胞接种:H358/A549细胞接种于6孔板盖玻片(2×10⁴个/孔),用含10% FBS的RPMI 1640培养基过夜培养。 2. 处理:细胞用Deltarasin(5~50 nM)处理24小时,4%多聚甲醛固定(室温15分钟),0.1% Triton X-100透化(10分钟)。 3. 染色与成像:5% BSA封闭1小时,抗KRAS一抗(1:500)4℃孵育过夜,Alexa Fluor 488偶联二抗(1:1000)室温孵育1小时;DAPI染核5分钟。共聚焦显微镜观察,ImageJ定量膜/胞质荧光强度比 [1] - 细胞增殖与信号检测实验[1]: 1. MTT增殖实验:细胞以5×10³个/孔接种96孔板,Deltarasin(1~50 nM)处理72小时。加20 μL MTT(5 mg/mL)孵育4小时,DMSO溶解甲瓒结晶,570 nm测吸光度。 2. Western blot实验:细胞以2×10⁵个/孔接种6孔板,Deltarasin(5~50 nM)处理24小时。含抑制剂的RIPA缓冲液裂解细胞,30 μg蛋白经10% SDS-PAGE分离,转移至PVDF膜,用抗p-ERK(1:1000)、抗p-AKT(1:1000)、抗总ERK/AKT(1:1000)及抗β-actin(1:5000)抗体孵育,ECL显影。 3. 克隆形成实验:细胞(200个/孔,6孔板)用Deltarasin(5~20 nM)处理14天,结晶紫染色,计数>50个细胞的克隆 [1] |
| 动物实验 |
Dissolved in 10% PLR and 5% DMSO; 10 mg/kg; Administered through i.p.
Mice bearing Panc-Tu-I xenografts H358 Xenograft Model (Literature 1): 1. Animal Preparation: Female BALB/c nu/nu mice (6–8 weeks old, 18–22g) were housed under SPF conditions (12h light/dark cycle, free access to food/water). 2. Tumor Induction: 5×10⁶ H358 cells (suspended in 0.2 mL PBS + 50% Matrigel) were subcutaneously injected into the right flank of each mouse. 3. Drug Preparation & Administration: Deltarasin was dissolved in 5% DMSO + 95% saline to concentrations of 1 mg/mL (10 mg/kg dose) and 2 mg/mL (20 mg/kg dose). When tumors reached ~100 mm³ (day 0), mice were randomized to groups (n=6/group) and received intraperitoneal injections once daily for 21 days (0.1 mL/10g body weight). Vehicle group received 5% DMSO + 95% saline. 4. Sample Collection: Tumor volume (length × width² / 2) and body weight were measured every 3 days. On day 21, mice were euthanized by cervical dislocation; tumors were excised (weighed, fixed in 10% formalin for immunohistochemistry or frozen for Western blot) [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
In Vitro Cytotoxicity (Literature 1):
Normal human cells (BEAS-2B bronchial epithelial cells, HEK293 embryonic kidney cells) were treated with Deltarasin (1–50 nM) for 72 hours. MTT assay showed cell viability >90% at all concentrations, indicating no significant cytotoxicity to normal cells [1] - In Vivo Safety (Literature 1): In the H358 xenograft model (Deltarasin 10–20 mg/kg, i.p., 21 days): - No mortality or abnormal behaviors (e.g., lethargy, anorexia) were observed; - Body weight change: 10 mg/kg group gained 3.2 ± 0.5g, 20 mg/kg group gained 2.8 ± 0.4g (vs. vehicle group 3.5 ± 0.6g), with weight loss <5%; - Serum biochemical parameters: ALT, AST, BUN, and creatinine levels were within normal ranges (no significant difference vs. vehicle); - Organ histopathology: Liver, kidney, and spleen tissues showed no inflammation, necrosis, or structural abnormalities (HE staining) [1] |
| 参考文献 | |
| 其他信息 |
Mechanism of Action (Literature 1):
Deltarasin binds specifically to the prenyl-binding pocket of PDEδ, preventing PDEδ from chaperoning prenylated KRAS to the plasma membrane. Reduced KRAS membrane localization inhibits downstream oncogenic signaling pathways (RAS-RAF-MEK-ERK, PI3K-AKT-mTOR), thereby suppressing proliferation and survival of KRAS-mutant cancer cells [1] - Therapeutic Potential (Literature 1): As a first-in-class inhibitor of the KRAS-PDEδ interaction, Deltarasin exhibits efficacy in KRAS-mutant lung cancer models (in vitro and in vivo), supporting its potential for treating KRAS-driven cancers (e.g., non-small cell lung cancer, pancreatic cancer) where KRAS mutations are frequent and refractory to traditional therapies [1] - Selectivity Advantage (Literature 1): Deltarasin shows no significant binding to other prenyl-binding proteins (e.g., PDE6δ, which is critical for visual function) or off-target kinases/enzymes, minimizing the risk of off-target side effects (e.g., visual disturbances) [1] |
| 分子式 |
C40H37N5O
|
|---|---|
| 分子量 |
603.75
|
| 精确质量 |
603.299
|
| CAS号 |
1440898-61-2
|
| 相关CAS号 |
Deltarasin hydrochloride;1613404-76-4;Deltarasin trihydrochloride;1440898-82-7
|
| PubChem CID |
73292904
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
824.3±75.0 °C at 760 mmHg
|
| 闪点 |
452.3±37.1 °C
|
| 蒸汽压 |
0.0±3.0 mmHg at 25°C
|
| 折射率 |
1.680
|
| LogP |
9.79
|
| tPSA |
56.9
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
46
|
| 分子复杂度/Complexity |
923
|
| 定义原子立体中心数目 |
1
|
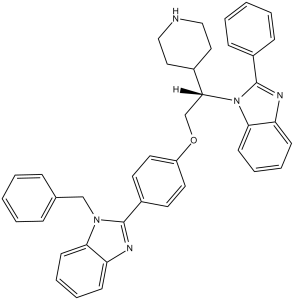
| SMILES |
C1CNCCC1[C@@H](COC2=CC=C(C=C2)C3=NC4=CC=CC=C4N3CC5=CC=CC=C5)N6C7=CC=CC=C7N=C6C8=CC=CC=C8
|
| InChi Key |
NCIOVAYUMQEQEU-VKZSUDIWSA-N
|
| InChi Code |
InChI=1S/C40H37N5O.3ClH/c1-3-11-29(12-4-1)27-44-36-17-9-7-15-34(36)42-39(44)32-19-21-33(22-20-32)46-28-38(30-23-25-41-26-24-30)45-37-18-10-8-16-35(37)43-40(45)31-13-5-2-6-14-31;;;/h1-22,30,38,41H,23-28H2;3*1H/t38-;;;/m1.../s1
|
| 化学名 |
2-[4-[(2S)-2-(2-phenyl-1H-benzimidazol-1-yl)-2-(4-piperidinyl)ethoxy]phenyl]-1-(phenylmethyl)-1H-benzimidazole, trihydrochloride
|
| 别名 |
Deltarasin hydrochloride; Deltarasin HCl; Deltarasin;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1.25 mg/mL (2.07 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 12.5 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 1.25 mg/mL (2.07 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 12.5 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 1.25 mg/mL (2.07 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6563 mL | 8.2816 mL | 16.5631 mL | |
| 5 mM | 0.3313 mL | 1.6563 mL | 3.3126 mL | |
| 10 mM | 0.1656 mL | 0.8282 mL | 1.6563 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|---|
|
|