| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|

| 靶点 |
Pentoxifylline: Tumor Necrosis Factor-α (TNF-α) (inhibitory activity), Nuclear Factor-kappa B (NF-κB) (suppressive effect), Phosphodiesterase 4 (PDE4) (weak inhibition) [1]
Pentoxifylline: Acts on pathways regulating red blood cell deformability, blood viscosity, and platelet aggregation [2] Pentoxifylline: Extracellular Signal-Regulated Kinase 1/2 (ERK1/2) and Protein Kinase B (AKT) (activating effect), Mammalian Target of Rapamycin (mTOR) [3] |
|---|---|
| 体外研究 (In Vitro) |
己酮可可碱以剂量依赖性方式抑制细胞生长(0.1–50 mM;24-48 小时)[3]。在 MDA-MB-231 细胞中,己酮可可碱(0.5 mM;12-36 小时)可减少自噬并促进细胞凋亡 [3]。在 MDA-MB-231 细胞中,己酮可可碱(0.5 mM;12-36 小时)可促进自噬 [3]。细胞周期的 G0/G1 期被己酮可可碱(0.5 mM;24-48 小时)阻断 [3]。己酮可可碱导致 LC3-II/LC3 比率升高 [3]。
1. 在人白细胞体外实验中,己酮可可碱呈浓度依赖性抑制细菌刺激诱导的促炎细胞因子TNF-α、IL-1β的产生,同时上调抗炎细胞因子IL-10的表达[1] 2. 己酮可可碱在体外可增加红细胞变形性,通过减少红细胞聚集降低全血粘度,并抑制ADP和胶原诱导的血小板聚集[2] 3. 在三阴性乳腺癌MDA-MB-231细胞中,己酮可可碱(0.5 mM)单独处理48小时可抑制42%的细胞增殖,诱导25%的细胞发生凋亡、25%的细胞发生自噬;与辛伐他汀(0.5 μM)联用时,细胞增殖抑制率达80%,凋亡率升高至>65%,自噬率降至<13%[3] 4. 己酮可可碱(0.5 mM)与辛伐他汀(0.5 μM)联用可使78%的MDA-MB-231细胞发生G0/G1期细胞周期阻滞,细胞克隆形成能力降至38±5%(单独使用己酮可可碱时为115±5%)[3] 5. 己酮可可碱(0.5 mM)可激活MDA-MB-231细胞中ERK1/2和AKT的磷酸化,抑制NF-κB信号通路,对mTOR的磷酸化无显著影响;联用处理还可升高细胞内活性氧(ROS)水平并增强caspase 3的活性[3] |
| 体内研究 (In Vivo) |
在暴露于短暂性全身缺血的大鼠中,己酮可可碱(200 mg/kg;腹膜内注射)发挥保护作用并减轻认知损伤[4]。
1. 己酮可可碱对下肢溃疡、银屑病、硬皮病等皮肤科疾病具有治疗效果,既可作为主要药物,也可作为辅助药物使用[1] 2. 外周血管疾病患者口服己酮可可碱(600–1200 mg/天,疗程至少6周)后,步行距离改善约100%,下肢静息痛、感觉异常症状缓解,肌肉血流量增加,下肢溃疡愈合加速,有效率达60–100%[2] 3. 脑血管疾病患者服用己酮可可碱(600–1200 mg/天,日本剂量为300–600 mg/天)后,约85%的患者获得显著临床改善,脑血流量(尤其是缺血区域)增加,神经运动功能缺损、言语障碍及主观症状得到缓解[2] 4. 己酮可可碱治疗外周血管疾病的疗效优于安慰剂、尼利地尔、腺苷和萘呋胺,治疗慢性脑血管疾病的效果优于氢化麦角碱、腺苷和吡硫醇[2] 5. 在大鼠短暂全脑缺血模型中,腹腔注射己酮可可碱(200 mg/kg,缺血前1小时和缺血后3小时给药)可显著改善Morris水迷宫实验中的海马依赖性空间记忆,减轻缺血诱导的认知损伤,海马CA1区锥体细胞数量与对照组无显著差异[4] |
| 酶活实验 |
1. 人白细胞细胞因子产生实验:从外周血中分离人白细胞,与细菌刺激物及不同浓度的己酮可可碱在适宜的培养基中共同孵育预设时间。孵育结束后收集培养上清,采用酶联免疫吸附试验(ELISA)检测TNF-α、IL-1β和IL-10的浓度,计算细胞因子产生的抑制率或促进率[1]
2. Caspase 3活性实验:将MDA-MB-231细胞用己酮可可碱单独或与辛伐他汀联合处理48小时后,用裂解液裂解细胞制备细胞裂解液。采用比色底物试剂盒检测caspase 3的活性,通过酶标仪检测405 nm处的吸光度值,反映caspase 3的相对活性[3] 3. 活性氧(ROS)检测实验:将MDA-MB-231细胞接种于培养板中,用ROS特异性荧光探针进行负载。负载完成后,用己酮可可碱和辛伐他汀处理细胞特定时间,通过流式细胞术检测细胞的荧光强度,定量细胞内ROS水平[3] |
| 细胞实验 |
细胞增殖测定[3]
细胞类型: MDA-MB-231 细胞 测试浓度: 0.1 mM、1 mM、5 mM、10 mM、 50 mM 孵育时间:24 小时、48 小时 实验结果:抑制细胞增殖剂量依赖性方式。 细胞凋亡分析[3] 细胞类型: MDA-MB-231 细胞 测试浓度: 0.5 mM 孵育时间:12 hrs(小时)、24 hrs(小时)、36 hrs(小时) 实验结果:诱导细胞凋亡。 自噬测定 [3] 细胞类型: MDA-MB-231 细胞 测试浓度: 0.5 mM 孵育持续时间:24 小时、48 小时 实验结果:大约 20-28% 的自噬被诱导。 细胞周期分析 [3] 细胞类型: MDA-MB-231 细胞 测试浓度: 0.5 mM 孵育持续时间:24 小时、48 小时 实验结果:诱导 G0/G1 期停滞。 蛋白质印迹分析[3] 细胞类型: MDA-MB-231 细胞 测试浓度: 0.5 mM 孵育持续时间:24小时、48小时 实验结果:诱导高LC3-II/LC3比率。 1. MTT细胞增殖实验:将MDA-MB-231细胞以每孔5×10³个的密度接种于96孔板,过夜培养。随后用己酮可可碱(0.5 mM)单独或与辛伐他汀(0.5 μM)联合处理细胞48小时。向每孔加入MTT溶液并孵育4小时,弃去上清后加入二甲基亚砜(DMSO)溶解甲臜结晶。用酶标仪检测570 nm处的吸光度,根据处理组和对照组的吸光度值计算细胞增殖抑制率[3] 2. 克隆形成实验:将MDA-MB-231细胞以低密度(每孔200个细胞)接种于6孔板,过夜培养。用己酮可可碱和辛伐他汀处理细胞,每3天更换一次培养基。培养14天后,用固定液固定克隆,结晶紫染色。计数含50个以上细胞的克隆数,计算克隆形成效率[3] 3. Annexin V/PI染色检测凋亡:将MDA-MB-231细胞用药物处理48小时后收集,用磷酸盐缓冲液(PBS)洗涤。按照染色方案用Annexin V-FITC和碘化丙啶(PI)对细胞进行染色,避光孵育15分钟。通过流式细胞术分析凋亡率,其中早期凋亡定义为Annexin V阳性/PI阴性,晚期凋亡/坏死定义为Annexin V阳性/PI阳性[3] 4. 细胞周期分析:将MDA-MB-231细胞用药物处理48小时后收集,用70%冷乙醇在4℃下固定过夜。固定后的细胞用PBS洗涤,加入含核糖核酸酶(RNase)的PI溶液染色,避光孵育30分钟。通过流式细胞术检测细胞周期分布(G0/G1、S、G2/M期),计算各期细胞的百分比[3] 5. 蛋白质印迹实验:将MDA-MB-231细胞用药物处理48小时后裂解,提取总细胞蛋白。蛋白样品经十二烷基硫酸钠-聚丙烯酰胺凝胶电泳(SDS-PAGE)分离后,转移至聚偏氟乙烯(PVDF)膜。用封闭液封闭膜后,依次孵育ERK1/2、磷酸化ERK1/2(p-ERK1/2)、AKT、磷酸化AKT(p-AKT)、mTOR、磷酸化mTOR(p-mTOR)和NF-κB的一抗及二抗。用化学发光底物显影蛋白条带,通过光密度法定量条带强度,并以内参蛋白进行归一化[3] 6. DNA片段化实验:采用DNA提取试剂盒从经己酮可可碱和辛伐他汀处理的MDA-MB-231细胞中提取基因组DNA。将提取的DNA经1.5%琼脂糖凝胶电泳分离,溴化乙锭染色后在紫外光下观察DNA片段化模式(梯状条带),验证凋亡的发生[3] 7. 自噬小体检测:将MDA-MB-231细胞用药物处理48小时后,用特异性标记自噬小体的荧光染料染色。在荧光显微镜下观察细胞,计数每个细胞的自噬小体数量;或通过流式细胞术检测荧光强度,定量自噬水平[3] |
| 动物实验 |
Animal/Disease Models: Adult male Wistar rats, 12-13 weeks old (250-300 g) [4]
Doses: 200 mg/kg Route of Administration: intraperitoneal (ip) injection, 1 hour before ischemia and 3 hrs (hrs (hours)) after ischemia. Experimental Results: Significant Improves spatial memory and memory abilities. The effect was Dramatically different from that of the sham operation group and the vehicle group. 1. Rat model of transient global cerebral ischemia: Thirty-two male Wistar rats (250–300 g) were randomly divided into four groups: control group, sham-operated group, vehicle group, and Pentoxifylline-treated group. Pentoxifylline was dissolved in a suitable vehicle , and administered intraperitoneally at a dose of 200 mg/kg (1 hour before ischemia and 3 hours after ischemia). Transient global cerebral ischemia was induced by bilateral common carotid artery occlusion for 20 minutes, followed by reperfusion. After reperfusion, the Morris Water Maze test was performed to evaluate spatial memory: the escape latency to find the hidden platform and the distance moved were recorded during the training phase, and the number of platform crossings and the time spent in the target quadrant were recorded during the probe trial. After the behavioral test, the rats were sacrificed, and brain sections were prepared and stained with Nissl staining to count the number of pyramidal cells in the hippocampal CA1 region [4] 2. Clinical trial for peripheral vascular disease: Patients with peripheral vascular disease were treated with oral controlled-release Pentoxifylline tablets at a dose of 600–1200 mg/day for at least 6 weeks. Clinical parameters including maximum walking distance, claudication distance, rest pain score, paresthesia symptoms, muscle blood flow (measured by Doppler ultrasound), and leg ulcer healing status were assessed at 2-week intervals during the treatment period [2] 3. Clinical trial for cerebrovascular disease: Patients with chronic cerebrovascular disorders received oral Pentoxifylline at a dose of 600–1200 mg/day (300–600 mg/day in Japan) for a long-term period (≥3 months). Cerebral blood flow was measured by single-photon emission computed tomography (SPECT) or computed tomography (CT) perfusion imaging, and neurological function was evaluated using a standardized neurological deficit score, including assessments of motor function, speech ability, and subjective symptoms such as dizziness and headache [2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Oral pentoxifylline (PTX) is almost completely absorbed but has low bioavailability of 20-30% due to extensive first-pass metabolism; three of the seven known metabolites, M1, M4, and M5 are present in plasma and appear soon after dosing. Single oral doses of 100, 200, and 400 mg of pentoxifylline in healthy males produced a mean tmax of 0.29-0.41 h, a mean Cmax of 272-1607 ng/mL, and a mean AUC0-∞ of 193-1229 ng\\*h/mL; corresponding ranges for metabolites 1, 4, and 5 were 0.72-1.15, 114-2753, and 189-7057. Single administration of a 400 mg extended-release tablet resulted in a heightened tmax of 2.08 ± 1.16 h, lowered Cmax of 55.33 ± 22.04 ng/mL, and a comparable AUC0-t of 516 ± 165 ng\\*h/mL; all these parameters were increased in cirrhotic patients. Smoking was associated with a decrease in the Cmax and AUCsteady-state of metabolite M1 but did not dramatically affect the pharmacokinetic parameters of pentoxifylline or other measured metabolites. Renal impairment increases the mean Cmax, AUC, and ratio to parent compound AUC of metabolites M4 and M5, but has no significant effect on PTX or M1 pharmacokinetics. Finally, similar to cirrhotic patients, the Cmax and tmax of PTX and its metabolites are increased in patients with varying degrees of chronic heart failure. Overall, metabolites M1 and M5 exhibit plasma concentrations roughly five and eight times greater than PTX, respectively. PTX and M1 pharmacokinetics are approximately dose-dependent, while those of M5 are not. Food intake before PTX ingestion delays time to peak plasma concentrations but not overall absorption. Extended-release forms of PTX extend the tmax to between two and four hours but also serves to ameliorate peaks and troughs in plasma concentration over time. Pentoxifylline is eliminated almost entirely in the urine and predominantly as M5, which accounts for between 57 and 65 percent of the administered dose. Smaller amounts of M4 are recovered, while M1 and the parent compound account for less than 1% of the recovered dose. The fecal route accounts for less than 4% of the administered dose. Pentoxifylline has a volume of distribution of 4.15 ± 0.85 following a single intravenous 100 mg dose in healthy subjects. Pentoxifylline given as a single 100 mg intravenous infusion has a clearance of 3.62 ± 0.75 L/h/kg in healthy subjects, which decreased to 1.44 ± 0.46 L/h/kg in cirrhotic patients. In another study, the apparent clearance of either 300 or 600 mg of pentoxifylline given intravenously (median and range) was 4.2 (2.8-6.3) and 4.1 (2.3-4.6) L/min, respectively. It is important to note that, due to the reversible extra-hepatic metabolism of the parent compound and metabolite 1, the true clearance of pentoxifylline may be even higher than the measured values. Metabolism / Metabolites Pentoxifylline (PTX) metabolism is incompletely understood. There are seven known metabolites (M1 through M7), although only M1, M4, and M5 are detected in plasma at appreciable levels, following the general pattern M5 > M1 > PTX > M4. As PTX apparent clearance is higher than hepatic blood flow and the AUC ratio of M1 to PTX is not appreciably different in cirrhotic patients, it is clear that erythrocytes are the main site of PTX-M1 interconversion. However, the reaction likely occurs in the liver as well. PTX is reduced in an NADPH-dependent manner by unknown an unidentified carbonyl reductase to form either [lisofylline] (the (R)-M1 enantiomer) or (S)-M1; the reaction is stereoselective, producing (S)-M1 exclusively in liver cytosol, 85% (S)-M1 in liver microsomes, and a ratio of 0.010-0.025 R:S-M1 after IV or oral dosing in humans. Although both (R)- and (S)-M1 can be oxidized back into PTX, (R)-M1 can also give rise to M2 and M3 in liver microsomes. _In vitro_ studies suggest that CYP1A2 is at least partly responsible for the conversion of [lisofylline] ((R)-M1) back into PTX. Unlike the reversible oxidation/reduction of PTX and its M1 metabolites, M4 and M5 are formed via irreversible oxidation of PTX in the liver. Studies in mice recapitulating the PTX-ciprofloxacin drug reaction suggest that CYP1A2 is responsible for the formation of M6 from PTX and of M7 from M1, both through de-methylation at position 7. In general, metabolites M2, M3, and M6 are formed at very low levels in mammals. Pentoxifylline is a known human metabolite of lisofylline. Biological Half-Life Overall, pentoxifylline has an elimination half-life of between 0.39 and 0.84 hours, while its primary metabolites have elimination half-lives of between 0.96 and 1.61 hours. 1. Absorption: Pentoxifylline is rapidly absorbed after oral administration in humans, with peak plasma concentrations (Cmax) reached at 1–2 hours; due to extensive first-pass metabolism in the liver, the oral bioavailability is only about 10–20% [2] 2. Distribution: Pentoxifylline has a high plasma protein binding rate of approximately 98% in humans; it distributes well in various tissues, with higher concentrations in ischemic areas of the brain and peripheral vascular tissues compared with normal tissues [2] 3. Metabolism: Pentoxifylline is mainly metabolized in the liver by cytochrome P450 (CYP) enzymes, and two major active metabolites are produced: 1-(5-hydroxyhexyl)-3,7-dimethylxanthine and 1-(3-carboxypropyl)-3,7-dimethylxanthine [2] 4. Elimination: The terminal half-life (t1/2) of Pentoxifylline in humans is about 0.5–1.5 hours; approximately 90% of the metabolites are excreted through the kidneys within 24 hours, and only a small amount of the parent drug is excreted unchanged [2] 5. Pharmacokinetic parameters (human, oral 600 mg): Cmax ≈ 1 μg/mL, Tmax = 1–2 hours, area under the plasma concentration-time curve (AUC0-∞) ≈ 2 μg·h/mL [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Chronic therapy with pentoxifylline has not been associated with elevations in serum enzyme levels, although the rigor with which liver test abnormalities were sought in patients taking the drug was not always clear. Despite its use for more than 3 decades, pentoxifylline has been linked to only rare and not completely convincing cases of clinically apparent liver injury. Nevertheless, adverse effects of hepatitis, jaundice, cholestasis and increased liver enzymes are listed in product labels for pentoxifylline. In reported cases, the time to onset was 3 to 4 weeks and the pattern of liver enzyme elevations was distinctly cholestatic (Case 1). Autoimmune and immunoallergic features were not present. The injury was self-limited and there have been no reports of acute liver failure, chronic hepatitis or vanishing bile duct syndrome associated with pentoxifylline therapy. In addition, pentoxifylline has been evaluated as the therapy of several liver diseases including acute alcoholic hepatitis and cirrhosis, nonalcoholic fatty liver disease and autoimmune liver conditions with varying results. In several small controlled trials in severe acute alcoholic hepatitis, pentoxifylline therapy was associated with a significant decrease in short term mortality and in the frequency of the hepatorenal syndrome. However, in large well controlled trials in alcoholic fatty liver, pentoxifylline with or without corticosteroids was found to have no effect on either short- or long-term mortality and minimal or no effect on rates of renal failure. Pentoxifylline has also been reported to improve serum aminotransferase levels and hepatic histology in adult patients with nonalcoholic steatohepatitis (NASH), but these findings have yet to be tested in larger randomized controlled trials. All studies, however, found pentoxifylline well tolerated in patients with liver disease and without evidence of hepatotoxicity. Likelihood score: D (possible rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Limited data indicate that pentoxifylline is poorly excreted into breastmilk. It would not be expected to cause any adverse effects in breastfed infants, especially if the infant is older than 2 months. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Pentoxifylline is approximately 45% bound to erythrocyte membranes. 1. Acute toxicity: The median lethal dose (LD50) of Pentoxifylline is approximately 2000 mg/kg (oral administration) in rats and 1000 mg/kg (intraperitoneal administration) in mice [2] 2. Chronic toxicity: In long-term clinical use (600–1200 mg/day for several weeks to months), Pentoxifylline is well-tolerated, and no significant histopathological changes have been observed in the liver, kidney, heart, brain or other major organs [2] 3. Adverse effects: Gastrointestinal symptoms (nausea, vomiting, diarrhea) are the most common adverse effects, occurring in about 3% of patients; these symptoms are mild and usually do not require discontinuation of the drug [1,2] 4. Drug-drug interaction: Pentoxifylline does not have significant interactions with commonly used drugs such as anticoagulants (e.g., warfarin), antihypertensives (e.g., beta-blockers) and lipid-lowering drugs (e.g., statins); it does not inhibit or induce the activity of major CYP450 isoenzymes at therapeutic doses [2] 5. Plasma protein binding: As described in the ADME section, the plasma protein binding rate of Pentoxifylline in humans is about 98%, and it does not significantly displace other drugs bound to plasma proteins [2] |
| 参考文献 |
|
| 其他信息 |
Pharmacodynamics
Pentoxifylline, a synthetic dimethylxanthine derivative structurally related to [theophylline] and [caffeine], exhibits hemorheological, anti-oxidative, and anti-inflammatory properties and is traditionally indicated in the treatment of peripheral arterial disease (PAD). In PAD patients with concurrent cerebrovascular and coronary artery diseases, pentoxifylline treatment has occasionally been associated with angina, arrhythmia, and hypotension. Concurrent use with [warfarin] should be associated with more frequent monitoring of prothrombin times. Also, patients with risk factors complicated by hemorrhages, such as retinal bleeding, peptic ulceration, and recent surgery, should be monitored periodically for bleeding signs. 1. Pentoxifylline is a methylxanthine derivative with hemorheological, anti-inflammatory and vasoactive properties [1,2] 2. The main mechanisms of action of Pentoxifylline include increasing red blood cell deformability, reducing blood viscosity, inhibiting platelet aggregation and thrombus formation, suppressing the production of pro-inflammatory cytokines (TNF-α, IL-1β) and the activation of the NF-κB signaling pathway, and activating the ERK1/2-AKT signaling pathway [1,2,3] 3. Dermatological applications: Pentoxifylline is a safe and cost-effective drug for the treatment of leg ulcers, psoriasis, scleroderma and other dermatological diseases, and can be used alone or in combination with other drugs (e.g., corticosteroids for psoriasis) [1] 4. Clinical indications: Pentoxifylline is approved for the treatment of peripheral vascular disease (such as intermittent claudication) and chronic cerebrovascular disease; preliminary studies have shown its potential efficacy in the treatment of vaso-occlusive crises of sickle cell disease, hearing disorders, ocular circulatory disorders, high altitude sickness, asthenozoospermia, and triple-negative breast cancer (in combination with simvastatin) [2,3] 5. Neuroprotective effect: Pentoxifylline has a protective effect on cognitive function in a rat model of transient global cerebral ischemia, but does not show a significant protective effect on hippocampal CA1 pyramidal cells [4] 6. Formulation and dosage: Pentoxifylline is available as conventional tablets and controlled-release tablets for oral administration; the recommended daily dose for adults is 600–1200 mg, administered in divided doses (200–400 mg three times a day) [2] |
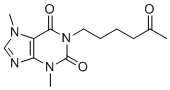
| 分子式 |
C13H18N4O3
|
|---|---|
| 分子量 |
278.30702
|
| 精确质量 |
278.137
|
| CAS号 |
6493-05-6
|
| 相关CAS号 |
Pentoxifylline-d6;1185878-98-1;Pentoxifylline-d5;1185995-18-9
|
| PubChem CID |
4740
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
531.3±56.0 °C at 760 mmHg
|
| 熔点 |
98-100°C
|
| 闪点 |
275.1±31.8 °C
|
| 蒸汽压 |
0.0±1.4 mmHg at 25°C
|
| 折射率 |
1.621
|
| LogP |
0.32
|
| tPSA |
78.89
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
20
|
| 分子复杂度/Complexity |
426
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
BYPFEZZEUUWMEJ-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C13H18N4O3/c1-9(18)6-4-5-7-17-12(19)10-11(14-8-15(10)2)16(3)13(17)20/h8H,4-7H2,1-3H3
|
| 化学名 |
1,2,3,6-Tetrahydro-3,7-dimethyl-1-(5-oxohexyl)-2,6-purindion
|
| 别名 |
Dimethyloxohexylxanthine; EHT-0202, EHT0202, EHT 0202; BL 191, BL191, BL-191; Oxpentifylline, Pentoxifilina, Theobromine, Trental, Vazofirin, Etazolate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ~93.3 mg/mL (~335.24 mM)
DMSO : ≥ 2.8 mg/mL (~10.06 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 110 mg/mL (395.24 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.5931 mL | 17.9656 mL | 35.9312 mL | |
| 5 mM | 0.7186 mL | 3.5931 mL | 7.1862 mL | |
| 10 mM | 0.3593 mL | 1.7966 mL | 3.5931 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Pentoxifylline Dose Optimization in Neonatal Sepsis
CTID: NCT04152980
Phase: Phase 3 Status: Completed
Date: 2024-08-09