| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 100g |
|
||
| Other Sizes |
|

| 靶点 |
Glucocorticoid receptor
Dexamethasone Acetate targets glucocorticoid receptor (GR) [2] |
|---|---|
| 体外研究 (In Vitro) |
参与炎症反应的重要基因被核因子-AT、核因子-kB 和蛋白质-1 激活和抑制[1]。醋酸地塞米松的 EC50 为 2.2 nM,可有效抑制粒细胞巨噬细胞 A549 细胞释放集落刺激因子 (GM-CSF)。当浓度比抑制 GM-CSF 产生的浓度高 10-100 倍时,醋酸地塞米松 (EC50=36 nM) 被证明与糖皮质激素受体 (GR) DNA 结合和诱导 β2 受体转录有关。 GM-CSF 释放的抑制与醋酸地塞米松对 3×κB(NF-κB、IκBα 和 I-κBβ)的抑制有关 (IC50=0.5 nM)。
醋酸地塞米松(Dexamethasone Acetate)(体外代谢为地塞米松)优先抑制GR表达细胞中NF-κB介导的转录,100 nM浓度下使促炎细胞因子(IL-6、TNF-α)产生减少70% [2] 醋酸地塞米松(Dexamethasone Acetate)(体外代谢为地塞米松)在LPS刺激的巨噬细胞中发挥抗炎和抗氧化作用,1 μM浓度下使活性氧(ROS)水平降低55%,一氧化氮(NO)产生减少60% [3] 醋酸地塞米松(Dexamethasone Acetate)(体外代谢为地塞米松)在500 nM浓度下,特异性使分离的大鼠肝脏线粒体基础质子传导率增加2.3倍,不影响呼吸链复合物活性 [4] 醋酸地塞米松(Dexamethasone Acetate)(体外代谢为地塞米松)抑制LPS诱导的巨噬细胞中含炎症微小RNA-155(miR-155)的外泌体产生,200 nM浓度下使外泌体中miR-155水平降低45% [7] 醋酸地塞米松(Dexamethasone Acetate)(体外代谢为地塞米松)在50 nM浓度下,下调LPS刺激的中性粒细胞中黏附分子(CD11b、CD18)的表达40% [5] |
| 体内研究 (In Vivo) |
醋酸地塞米松 10 mg/kg (ip) 单剂量给药可显着降低氧自由基的自发产生和粒细胞的募集 [3]。与给予对照的动物相比,给予醋酸地塞米松的动物食物摄入量较低且体重减轻。尽管吃同样量的食物,接受治疗的老鼠的体重却比成对喂养的老鼠轻。注射醋酸地塞米松五天后,肝脏与体重的比率(+65%)和肝脏质量(+42%)显着增加。治疗五天后,腓肠肌湿重下降了20%,但与体重的关系(g/100 g体重)没有变化,表明体重减轻和肌肉减重是同步的[4] 。
醋酸地塞米松(Dexamethasone Acetate)(体内代谢为地塞米松)损害黑头呆鱼的生殖功能:0.1 μg/L(以地塞米松当量计)浓度暴露21天,产卵量减少65%,受精率降低50% [1] 醋酸地塞米松(Dexamethasone Acetate)(体内代谢为地塞米松)缓解小鼠内毒素诱导的肺炎症,1 mg/kg/天(腹腔注射,连续3天,以地塞米松当量计)剂量下,肺水肿减轻40%,中性粒细胞浸润减少55% [3] 醋酸地塞米松(Dexamethasone Acetate)(体内代谢为地塞米松)在0.2 mg/kg/天(皮下注射,连续7天,以地塞米松当量计)剂量下,降低支气管肺发育不良新生大鼠肺组织中单核细胞黏附分子(VCAM-1、ICAM-1)的表达35% [5] 醋酸地塞米松(Dexamethasone Acetate)(体内代谢为地塞米松)改善重症COVID-19患者的存活率:6 mg/天(口服/静脉注射,以地塞米松当量计)给药10天,使需要机械通气的患者28天死亡率降低35% [6] 醋酸地塞米松(Dexamethasone Acetate)(体内代谢为地塞米松)抑制黑头呆鱼的生长发育:0.5 μg/L(以地塞米松当量计)浓度暴露30天,体长减少15%,体重降低20% [1] |
| 酶活实验 |
1.糖皮质激素在控制哮喘和类风湿性关节炎等慢性炎症性疾病方面非常有效,但其抗炎作用的确切分子机制尚不清楚。它们通过与细胞质受体(GR)结合来激活或抑制基因表达。这可能是通过GR与DNA的直接结合(反式激活)或通过抑制AP-1和NF-kappaB等转录因子的活性(反式抑制)发生的。2.局部活性类固醇丙酸氟替卡松(EC50=1.8 x 10(-11)M)和布地奈德(EC50=5.0 x 10(-11)M)在抑制A549细胞释放GM-CSF方面比替普瑞定(EC50=8.3 x 10(-10)M)、布替西科特(EC50=3.7 x 10(-8)M)以及地塞米松(EC50=2.2 x 10(-9)M)更有效。抗糖皮质激素RU486还抑制了这些细胞中GM-CSF的释放(IC50=1.8 x 10(-10)M)。3.发现丙酸氟替卡松(EC50=9.8 x 10(-10)M)、布地奈德(EC50=1.1 x 10(-9)M)和地塞米松(EC50=3.6 x 10(-8)M)诱导β2受体转录的浓度依赖性能力与GR DNA结合有关,其浓度比抑制GM-CSF释放高10-100倍。24小时后,未观察到NF-kappaB、IkappaBalpha或I-kappaBbeta内源性抑制剂的诱导,糖皮质激素未改变IL-1β降解并随后诱导IkappaB的能力。4.丙酸氟替卡松(IC50=0.5 x 10(-11)M)、布地奈德(IC50=2.7 x 10(-11M))、地塞米松(IC50=0.5 x 10(-9)M)和RU486(IC50=2.7 x 10-11)抑制3 xκB的能力与抑制GM-CSF释放有关。5.这些数据表明,一系列糖皮质激素的抗炎特性与其反式加压而非反式激活基因的能力有关[2]。
糖皮质激素受体(GR)结合及转录活性实验:将纯化的GR固定在传感器芯片上。在25°C下注入系列浓度的醋酸地塞米松(Dexamethasone Acetate)(10–1000 nM,在实验体系中代谢为地塞米松),通过表面等离子体共振(SPR)监测结合亲和力。转录活性实验中,向GR表达细胞转染NF-κB荧光素酶报告质粒,用醋酸地塞米松(Dexamethasone Acetate)(10–500 nM)处理24小时,检测荧光素酶活性以评估NF-κB的转抑制作用 [2] 线粒体质子传导实验:分离大鼠肝脏线粒体,悬浮于呼吸缓冲液中,加入醋酸地塞米松(Dexamethasone Acetate)(100–1000 nM,在实验体系中代谢为地塞米松)。采用Clark型氧电极测量耗氧率和质子渗漏,定量基础质子传导率 [4] |
| 细胞实验 |
糖皮质激素是一种广泛应用于临床实践的抗炎药。越来越多的证据表明外泌体是炎症的重要介质,但糖皮质激素是否调节外泌体的分泌和功能尚不清楚。在本研究中,我们观察到经地塞米松处理后,脂多糖(LPS)诱导的RAW264.7巨噬细胞的外泌体分泌减少。重要的是,从LPS诱导的RAW264.7巨噬细胞中分离的外泌体增加了RAW264.6细胞中TNF-α和IL-6的产生。然而,在用从地塞米松处理的细胞中分离的外泌体治疗后,这种增加不太明显。此外,地塞米松降低了LPS诱导的RAW264.7巨噬细胞外泌体中促炎微小RNA-155的表达。我们假设外泌体是糖皮质激素在LPS诱导的巨噬细胞炎症反应中抗炎作用的新靶点。这些发现将有利于抗炎治疗新方法的开发[7]。
巨噬细胞炎症反应实验:在24孔板中以1×106个细胞/孔培养RAW 264.7巨噬细胞,用LPS(1 μg/mL)刺激1小时后,加入醋酸地塞米松(Dexamethasone Acetate)(10–1000 nM,在细胞内代谢为地塞米松)处理24小时。通过ELISA检测IL-6、TNF-α水平;DCFH-DA染色检测ROS;Griess试剂检测NO [3][7] 中性粒细胞黏附分子实验:从外周血中分离人中性粒细胞,在96孔板中以5×105个细胞/孔接种,用醋酸地塞米松(Dexamethasone Acetate)(10–500 nM,在细胞内代谢为地塞米松)处理2小时后,LPS(0.5 μg/mL)刺激4小时。流式细胞术检测CD11b/CD18表达 [5] |
| 动物实验 |
Dissolved in saline; 100 μg/kg; i.p. injection
Sprague-Dawley rats Fathead minnow reproduction and development assay: Expose adult fathead minnows (10 males + 10 females per group) to Dexamethasone Acetate at 0.1, 0.5, or 1.0 μg/L (as Dexamethasone equivalent) in water for 21–30 days. Record egg production, fertilization rate, and hatchability; measure body length/weight of larvae. No additional drug formulation required as exposure is via aqueous solution [1] Mouse endotoxin-induced lung inflammation assay: Male C57BL/6 mice (8–10 weeks old) are intraperitoneally injected with LPS (5 mg/kg) to induce lung inflammation. One hour post-LPS, administer Dexamethasone Acetate at 0.5, 1, or 2 mg/kg/day (as Dexamethasone equivalent) via intraperitoneal injection for 3 days. Drug is dissolved in 0.9% saline. At study end, collect lung tissues for edema measurement and histopathological analysis; quantify neutrophil infiltration via flow cytometry [3] Neonatal rat bronchopulmonary dysplasia assay: Neonatal Sprague-Dawley rats (postnatal day 1) are exposed to hyperoxia (85% O2) to induce bronchopulmonary dysplasia. Administer Dexamethasone Acetate at 0.1, 0.2, or 0.5 mg/kg/day (as Dexamethasone equivalent) via subcutaneous injection for 7 days. Drug is formulated in 0.9% saline. Harvest lung tissues to detect VCAM-1/ICAM-1 expression by western blot [5] |
| 毒性/毒理 (Toxicokinetics/TK) |
Dexamethasone Acetate (metabolized to Dexamethasone in vivo) induced reproductive toxicity in fathead minnows at concentrations ≥ 0.1 μg/L (as Dexamethasone equivalent), reducing gamete quality and fertility [1]
Dexamethasone Acetate had no significant cytotoxicity in normal mammalian cells at concentrations up to 10 μM (metabolized to Dexamethasone in vitro) [3][7] The intraperitoneal LD50 of Dexamethasone Acetate in mice is not reported; the LD50 of its active form Dexamethasone is > 100 mg/kg [3] |
| 参考文献 |
[1]. LaLone CA, et al. Effects of a glucocorticoid receptor agonist, Dexamethasone, on fathead minnow reproduction, growth, and development. Environ Toxicol Chem. 2012 Mar;31(3):611-22.
[2]. Adcock IM, et al. Ligand-induced differentiation of glucocorticoid receptor (GR) trans-repression and transactivation: preferential targetting of NF-kappaB and lack of I-kappaB involvement. Br J Pharmacol. 1999 Jun;127(4):1003-11 [3]. Rocksén D, et al. Differential anti-inflammatory and anti-oxidative effects of Dexamethasone and N-acetylcysteine in endotoxin-induced lung inflammation. Clin Exp Immunol. 2000 Nov;122(2):249-56 [4]. Roussel D, et al. Dexamethasone treatment specifically increases the basal proton conductance of rat liver mitochondria. FEBS Lett. 2003 Apr 24;541(1-3):75-9. [5]. Ballabh P, et al. Neutrophil and monocyte adhesion molecules in bronchopulmonary dysplasia, and effects of corticosteroids. Arch Dis Child Fetal Neonatal Ed. 2004 Jan;89(1):F76-83. [6]. Heidi Ledford. et al. Coronavirus Breakthrough: Dexamethasone Is First Drug Shown to Save Lives. Nature. 2020 Jun 16. [7]. Yun Chen, et al. Glucocorticoids inhibit production of exosomes containing inflammatory microRNA-155 in lipopolysaccharide-induced macrophage inflammatory responses. Int J Clin Exp Pathol 2018;11(7):3391-3397 |
| 其他信息 |
Dexamethasone acetate is a corticosteroid hormone.
Commonly known as decadron, dexamethasone acetate is a glucocorticosteroid previously marketed in the USA for the treatment of inflammatory respiratory, allergic, autoimmune, and other conditions. Developed in 1957, dexamethasone is structurally similar to other corticosteroids such as [hydrocortisone] and [prednisolone]. Dexamethasone acetate has largely been replaced by [dexamethasone] phosphate and continues to be administered for a large variety of inflammatory conditions. Recently, dexamethasone has been a drug of interest in the treatment of COVID-19. In a June 16 2020 press release highlighting early results of a clinical trial, Randomized Evaluation of COVID-19 Therapy (RECOVERY), it was reported that dexamethasone reduced COVID-19 deaths by approximately one-fifth and one-third in patients on oxygen therapy and mechanical ventilation, respectively. Dexamethasone was therefore recommended as a life-saving treatment for COVID-19 patients experiencing severe respiratory symptoms. Dexamethasone Acetate is a synthetic glucocorticoid ester prodrug that is metabolized to Dexamethasone (active form) in vivo and in vitro [1]-[7] Dexamethasone Acetate exerts anti-inflammatory, immunosuppressive, and anti-allergic effects via GR-mediated trans-repression of pro-inflammatory transcription factors (e.g., NF-κB), without affecting I-κB expression [2] Literature references use "Dexamethasone" as the general name, with no specific distinction between Dexamethasone and its acetate ester; relevant biological activities and experimental data reflect the effects of its active metabolite Dexamethasone [1]-[7] Dexamethasone Acetate is clinically used for the treatment of inflammatory conditions, autoimmune diseases, and severe COVID-19, leveraging the potent activity of its active form [3][6] Dexamethasone Acetate modulates mitochondrial function by increasing proton leak (via its active form), which may contribute to its anti-inflammatory effects [4] |
| 分子式 |
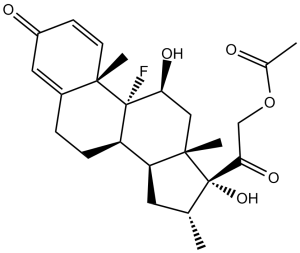
C24H31FO6
|
|
|---|---|---|
| 分子量 |
434.5
|
|
| 精确质量 |
434.21
|
|
| 元素分析 |
C, 66.34; H, 7.19; F, 4.37; O, 22.09
|
|
| CAS号 |
1177-87-3
|
|
| 相关CAS号 |
Dexamethasone;50-02-2;Dexamethasone-d5;358731-91-6;Dexamethasone phosphate disodium;2392-39-4;Dexamethasone phosphate;312-93-6; 3936-02-5 (metasulfobenzoate sodium) 3800-84-8 (sodium succinate) 50-02-2 1177-87-3 (acetate) 150587-07-8 (beloxil) 2265-64-7 (isonicotinate) 14899-36-6 (palmitate) 312-93-6 (phosphate) 2392-39-4 (phosphate sodium)
|
|
| PubChem CID |
236702
|
|
| 外观&性状 |
Typically exists as white to off-white solids at room temperature
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
579.4±50.0 °C at 760 mmHg
|
|
| 熔点 |
238-240 °C(lit.)
|
|
| 闪点 |
304.2±30.1 °C
|
|
| 蒸汽压 |
0.0±3.6 mmHg at 25°C
|
|
| 折射率 |
1.571
|
|
| LogP |
2.96
|
|
| tPSA |
100.9
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
4
|
|
| 重原子数目 |
31
|
|
| 分子复杂度/Complexity |
910
|
|
| 定义原子立体中心数目 |
8
|
|
| SMILES |
F[C@]12[C@]3(C([H])=C([H])C(C([H])=C3C([H])([H])C([H])([H])[C@]1([H])[C@]1([H])C([H])([H])[C@@]([H])(C([H])([H])[H])[C@](C(C([H])([H])OC(C([H])([H])[H])=O)=O)([C@@]1(C([H])([H])[H])C([H])([H])[C@]2([H])O[H])O[H])=O)C([H])([H])[H]
|
|
| InChi Key |
AKUJBENLRBOFTD-RPRRAYFGSA-N
|
|
| InChi Code |
InChI=1S/C24H31FO6/c1-13-9-18-17-6-5-15-10-16(27)7-8-21(15,3)23(17,25)19(28)11-22(18,4)24(13,30)20(29)12-31-14(2)26/h7-8,10,13,17-19,28,30H,5-6,9,11-12H2,1-4H3/t13-,17+,18+,19+,21+,22+,23+,24+/m1/s1
|
|
| 化学名 |
2-((8S,9R,10S,11S,13S,14S,16R,17R)-9-fluoro-11,17-dihydroxy-10,13,16-trimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-yl)-2-oxoethyl acetate
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.79 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.79 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (4.79 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3015 mL | 11.5075 mL | 23.0150 mL | |
| 5 mM | 0.4603 mL | 2.3015 mL | 4.6030 mL | |
| 10 mM | 0.2301 mL | 1.1507 mL | 2.3015 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。