| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| Other Sizes |
|
| 靶点 |
Nrf2
|
|---|---|
| 体外研究 (In Vitro) |
富马酸二甲酯(DMF;20–200 μM;24 小时)显着降低 SGC-7901、HT29、HCT116 和 CT26 的活力 [1]。在 CT26 细胞中,富马酸二甲酯(DMF;100 μM;3-24 小时)可显着激活 p38、ERK 和 JNK[1]。富马酸二甲酯通过减少涉及 GSH 消耗、提高 ROS 和刺激 MAPK 介导的信号传导的炎症转导途径发挥作用 [1]。通过降低 MHC II 类、CD80 和 CD86 的表达以及炎症细胞因子(IL-12 和 IL-6)的合成,富马酸二甲酯可防止树突状细胞 (DC) 发育。 Dimethyl fumarate 通过阻断 NF-κB 和 ERK1/2-MSK1 信号传导来减少 DC 成熟和累积 Th1 和 Th17 细胞分泌。富马酸二甲酯还会损害 p65 核转位和磷酸化 [2]。富马酸二甲酯 (DMF),一种免疫抗氧化反应细胞活力测定 [1]
|
| 体内研究 (In Vivo) |
富马酸二甲酯(DMF;50 mg/kg;每天;持续 7 天)被证明可以上调 Nrf2 调节的细胞保护基因的 mRNA 和蛋白质水平,并减少 6-OHDA 诱导的 C57BL 皮纹。八周大的雄性 C57BL/6 小鼠作为身体氧化的动物模型 [4]。
|
| 细胞实验 |
细胞活力测定[1]
细胞类型: SGC-7901、HT29、HCT116 和 CT26 细胞 测试浓度: 20 μM、50 μM、100 μM ,200 μM 孵育调节剂和诱导剂,抑制 HIV 复制和神经毒素释放 [3]。 孵育持续时间:24 小时 实验结果:SGC-7901、HT29、HCT116 和 CT26 癌细胞的细胞活力降低。 蛋白质印迹分析 [1] 细胞类型: CT26 癌细胞 测试浓度: 100 μM 孵育持续时间:3 hrs(小时)、6 hrs(小时)、12 hrs(小时)、24 hrs(小时) 实验结果:治疗3至24小时后,CT26细胞中的JNK、p38和ERK显着激活。 |
| 动物实验 |
Animal/Disease Models: Male C57BL/6 mice (8weeks old)[4]
Doses: 50 mg/kg Route of Administration: po (oral gavage); daily; for 7 days Experimental Results:Was shown to upregulate mRNA and protein levels of Nrf2 and Nrf2-regulated cytoprotective genes. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Once ingested, dimethyl fumarate is rapidly hydrolyzed by esterases to form monomethyl fumarate (MMF). Therefore, there is a negligible amount of dimethyl fumarate in the body, and all pharmacokinetic information is quantified with MMF. The time to maximum concentration (tmax) of MMF ranges between 2 and 2.5 hours. In patients with multiple sclerosis given 240 mg of dimethyl fumarate two times a day with food, the Cmax and AUC were 1.87 mg/L and 8.21 mg⋅hr/L, respectively. High-fat, high-calorie meals decrease the Cmax of MMF by 40% and cause a tmax delay from 2 hours to 5.5 hours; however, these changes are not considered clinically significant. The main route of elimination of dimethyl fumarate is by CO2 exhalation, which accounts for 60% of the dose. The other minor routes of elimination are through the kidney (16% of the dose) and feces (1% of the dose). Trace amounts of unchanged monomethyl fumarate (the active metabolite of dimethyl fumarate) are present in urine. In healthy people, monomethyl fumarate (MMF) has a variable volume of distribution of 53 to 73 litres. Monomethyl fumarate (MMF), the active metabolite of dimethyl fumarate, has a rapid clearance. Its apparent clearance (Cl/F) appears to be dose-independent. After oral administration of Tecfidera, dimethyl fumarate undergoes rapid presystemic hydrolysis by esterases and is converted to its active metabolite, monomethyl fumarate (MMF). Dimethyl fumarate is not quantifiable in plasma following oral administration of Tecfidera. Therefore all pharmacokinetic analyses related to Tecfidera were performed with plasma MMF concentrations. ... The median Tmax of MMF is 2-2.5 hours. The peak plasma concentration (Cmax) and overall exposure (AUC) increased approximately dose proportionally in the dose range studied (120 mg to 360 mg). Following administration of Tecfidera 240 mg twice a day with food, the mean Cmax of MMF was 1.87 mg/L and AUC was 8.21 mg.hr/L in MS patients. Exhalation of CO2 is the primary route of elimination, accounting for approximately 60% of the Tecfidera dose. Renal and fecal elimination are minor routes of elimination, accounting for 16% and 1% of the dose respectively. Trace amounts of unchanged monomethyl fumarate (MMF) were present in urine. The apparent volume of distribution of monomethyl fumarate (MMF) varies between 53 and 73 L in healthy subjects. Human plasma protein binding of MMF is 27-45% and independent of concentration. /Monomethyl fumarate, active metabolite/ Metabolism / Metabolites Dimethyl fumarate is quickly hydrolyzed by esterases in the gastrointestinal tract, tissues, and blood to form monomethyl fumarate (MMF), its active metabolite. MMF then undergoes subsequent metabolism through the tricarboxylic acid (TCA) cycle. The main metabolites of dimethyl fumarate are MMF, glucose, citric, and fumaric acid. Cytochrome P450 (CYP) enzymes do not participate in the metabolism of dimethyl fumarate. In humans, Tecfidera is extensively metabolized by esterases, which are ubiquitous in the gastrointestinal tract, blood and tissues, before it reaches the systemic circulation. Further metabolism occurs through the tricarboxylic acid (TCA) cycle, with no involvement of the cytochrome P450 (CYP) system. A single 240 mg (14)C-dimethyl fumarate dose study identified monomethyl fumarate, fumaric and citric acid, and glucose as the major metabolites in plasma. The downstream metabolism of fumaric and citric acid occurs through the TCA cycle, with exhalation of CO2 serving as a primary route of elimination. Less than 0.1% of the dose is excreted as unchanged dimethyl fumarate in urine. In humans, dimethyl fumarate is extensively metabolized by esterases, which are ubiquitous in the gastrointestinal tract, blood, and tissues, before it reaches the systemic circulation. Further metabolism of monomethyl fumarate (MMF) occurs through the tricarboxylic acid (TCA) cycle, with no involvement of the cytochrome P450 (CYP) system. MMF, fumaric and citric acid, and glucose are the major metabolites in plasma. Biological Half-Life The dimethyl fumarate metabolite monomethyl fumarate (MMF) has a short half-life of about 1 hour. MMF does not accumulate after repeated doses of dimethyl fumarate. The terminal half-life of monomethyl fumarate (MMF) is approximately 1 hour and no circulating MMF is present at 24 hours in the majority of individuals. /Monomethyl fumarate, active metabolite/ |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Dimethyl fumarate is a white to off-white powder formulated into delayed release capsules. It is used for the treatment of patients with relapsing forms of multiple sclerosis. Dimethyl fumarate is also used as a biocide to kill molds that may cause products such as furniture or shoes to deteriorate during storage or transportation in a humid climate. Placed in "Desiccant" sachets inside the furniture or footwear boxes, dimethyl fumarate evaporates and impregnates the product, protecting it from molds. HUMAN EXPOSURE AND TOXICITY: When used as a biocide, dimethyl fumarate has caused painful dermatitis. The fact that in serious cases the dermatitis is particularly difficult to treat adds to the damage. Dimethyl fumarate also has toxicity related to its use as a treatment for multiple sclerosis. A patient with multiple sclerosis who was being treated with dimethyl fumarate developed progressive multifocal leukoencephalopathy (PML), and later died. The patient who died was not taking any other drugs that affect the immune system or drugs that are thought to be associated with PML. Patients taking dimethyl fumarate should be advised to contact their clinician if they develop any symptoms that may be suggestive of PML. Treatment with dimethyl fumarate should not be initiated in patients with signs and symptoms of a serious infection. Dimethyl fumarate was clastogenic in the in vitro chromosomal aberration assay in human peripheral blood lymphocytes in the absence of metabolic activation. ANIMAL STUDIES: Acute toxicity studies were performed in mice and rats using oral and intraperitoneal routes. In mice, reduced motility, ataxia, dyspnea, cyanosis, muscular hypotonia were observed at oral doses as low as 681 mg/kg. Ataxia and hypopnea were observed at i.p. doses as low as 464 mg/kg. In rats, ataxia, muscular hypotonia, inhibited respiratory rate and motility were noted at oral doses as low as 2610 mg/kg. Reduced food intake and decreased body weight gain were seen at 1470 and 2150 mg/kg, respectively. Ataxia, muscular hypotonia, reduced motility and respiratory rate were also observed at intraperitoneal doses as low as 681 mg/kg. Dyspnea (825 mg/kg); tremor, pilo-erection (1000 mg/kg); abdominal positioning (1470 mg/kg) were also noted. In these studies, the kidneys, forestomach and liver were identified as target organs. In mice, oral administration of dimethyl fumarate (25, 75, 200, and 400 mg/kg/day) for up to two years resulted in an increase in nonglandular stomach (forestomach) and kidney tumors: squamous cell carcinomas and papillomas of the forestomach in males and females at 200 and 400 mg/kg/day; leiomyosarcomas of the forestomach at 400 mg/kg/day in males and females; renal tubular adenomas and carcinomas at 200 and 400 mg/kg/day in males; and renal tubule adenomas at 400 mg/kg/day in females. In rats, oral administration of dimethyl fumarate (25, 50, 100, and 150 mg/kg/day) for up to two years resulted in increases in squamous cell carcinomas and papillomas of the forestomach at all doses tested in males and females, and in testicular interstitial (Leydig) cell adenomas at 100 and 150 mg/kg/day. In rats administered dimethyl fumarate orally (25, 100, 250 mg/kg/day) throughout organogenesis, embryo fetal toxicity (reduced fetal body weight and delayed ossification) were observed at the highest dose tested. This dose also produced evidence of maternal toxicity (reduced body weight). Oral administration of dimethyl fumarate (25, 100, and 250 mg/kg/day) to rats throughout organogenesis and lactation resulted in increased lethality, persistent reductions in body weight, delayed sexual maturation (male and female pups), and reduced testicular weight at the highest dose tested. Neurobehavioral impairment was observed at all doses. In rabbits administered dimethyl fumarate orally (25, 75, and 150 mg/kg/day) throughout organogenesis, embryo lethality and decreased maternal body weight were observed at the highest dose tested. In male rats, oral administration of dimethyl fumarate (75, 250, and 375 mg/kg/day) prior to and throughout the mating period had no effect on fertility; however, increases in non-motile sperm were observed at the mid and high doses. In female rats, oral administration of dimethyl fumarate (20, 100, and 250 mg/kg/day) prior to and during mating and continuing to gestation day 7 caused disruption of the estrous cycle and increases in embryo lethality at the highest dose tested. Testicular toxicity (germinal epithelial degeneration, atrophy, hypospermia, and/or hyperplasia) was observed at clinically relevant doses in mice, rats, and dogs in subchronic and chronic oral toxicity studies of dimethyl fumarate. Dimethyl fumarate was not mutagenic in the in vitro bacterial reverse mutation (Ames) assay, and it was not clastogenic in the in vivo micronucleus assay in the rat. Toxicity Data LC (mouse) > 3,100 mg/m3/10min Interactions The bioreductive antitumor agent, mitomycin C (MMC), requires activation by reductive enzymes like NAD(P)H:quinone oxidoreductase 1 (NQO1). ...A novel approach /was used/ to increase MMC efficacy by selectively inducing NQO1 in tumor cells in vivo. CD-1 nude mice were implanted with HCT116 cells, and fed control diet or diet containing 0.3% of the NQO1 inducer, dimethyl fumarate (DMF). The mice were then treated with saline, 2.0, 3.5 or 2.0 mg/kg MMC and dicoumarol, an NQO1 inhibitor. The DMF diet increased NQO1 activity by 2.5-fold in the tumors, but had no effect in marrow cells. Mice given control diet/2.0 mg/kg MMC had tumors with the same volume as control mice; however, mice given DMF diet/2.0 mg/kg MMC had significantly smaller tumors. Tumor volumes in mice given DMF/2.0 mg/kg MMC were similar to those in mice given control diet/3.5 mg/kg MMC. Tumor inhibition was partially reversed in mice given DMF/2.0 mg/kg MMC and dicoumarol. DMF diet/2.0 mg/kg MMC treatment did not increase myelosuppression and did not produce any organ toxicity. These results provide strong evidence that dietary inducers of NQO1 can increase the antitumor activity of bioreductive agents like MMC without increasing toxicity. NQO1 is a reductive enzyme that is important for the activation of many bioreductive agents and is a target for an enzyme-directed approach to cancer therapy. It can be selectively induced in many tumor types by a number of compounds including dimethyl fumarate... . RH1 (2,5-diaziridinyl-3-(hydroxymethyl)- 6-methyl-1,4-benzoquinone) is a new bioreductive agent currently in clinical trials. ... HCT116 human colon cancer cells and T47D human breast cancer cells were incubated with or without dimethyl fumarate or sulforaphane followed by mitomycin C or RH1 treatment, and cytotoxic activity was measured by a clonogenic (HCT116) or MTT assay (T47D). Dimethyl fumarate and sulforaphane treatment increased NQO1 activity by 1.4- to 2.8-fold and resulted in a significant enhancement of the antitumor activity of mitomycin C, but not of RH1. This appeared to be due to the presence of a sufficient constitutive level of NQO1 activity in the tumor cells to fully activate the RH1. Mice were implanted with HL60 human promyelocytic leukemia cells, which have low levels of NQO1 activity. The mice were fed control or dimethyl fumarate-containing diet and were treated with RH1. NQO1 activity in the tumors increased but RH1 produced no antitumor activity in mice fed control or dimethyl fumarate diet. This is consistent with a narrow window of NQO1 activity between no RH1 activation and maximum RH1 activation. This study suggests that selective induction of NQO1 in tumor cells is not likely to be an effective strategy for enhancing the antitumor activity of RH1... ... The effects of butylated hydroxyanisole (BHA) /were/ compared with those of other inducers of DT-diaphorase. Rats were dosed with BHA, butylated hydroxytoluene (BHT), ethoxyquin (EQ), dimethyl fumarate (DMF) or disulfiram (DIS) and then challenged with a toxic dose of the naphthoquinones. All the inducers protected against the hemolytic anemia induced by 2-methyl-1,4-naphthoquinone in rats, with BHA, BHT and EQ being somewhat more effective than DMF and DIS. A similar order of activity was recorded in the relative ability of these substances to increase hepatic activities of DT-diaphorase, consistent with a role for this enzyme in facilitating conjugation and excretion of this naphthoquinone. In contrast, all the compounds increased the hemolytic activity of 2-hydroxy-1,4-naphthoquinone. DMF and DIS were significantly more effective in this regard than BHA, BHT and EQ. DMF and DIS also caused a much greater increase in levels of DT-diaphorase in the intestine, suggesting that 2-hydroxy-1,4-naphthoquinone is activated by this enzyme in the gut. BHA, BHT and EQ had no effect on the nephrotoxicity of 2-hydroxy-1,4-naphthoquinone, but the severity of the renal lesions was decreased in rats pre-treated with DMF and DIS. The results of the present experiments show that modulation of tissue levels of DT-diaphorase may not only alter the severity of naphthoquinone toxicity in vivo, but may also change the relative toxicity of these substances to different target organs. DT-diaphorase is a two-electron reducing enzyme that activates the bioreductive anti-tumour agent, mitomycin C (MMC). Cell lines having elevated levels of DT-diaphorase are generally more sensitive to MMC. ... DT-diaphorase can be induced in human tumor cells by a number of compounds, including 1,2-dithiole-3-thione. ... This study ... investigated whether induction of DT-diaphorase could enhance the cytotoxic activity of MMC in six human tumor cell lines representing four tumor types. DT-diaphorase was induced by many dietary inducers, including ... dimethyl fumarate ... .The cytotoxicity of MMC was significantly increased in four tumor lines with the increase ranging from 1.4- to threefold. In contrast, MMC activity was not increased in SK-MEL-28 human melanoma cells and AGS human gastric cancer cells, cell lines that have high base levels of DT-diaphorase activity. Toxicity to normal human marrow cells was increased by 50% when MMC was combined with 1,2-dithiole-3-thione, but this increase was small in comparison with the threefold increase in cytotoxicity to tumor cells. ... For more Interactions (Complete) data for DIMETHYL FUMARATE (9 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat oral 2240 mg/kg LD50 Rabbit dermal 1259 mg/kg |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Dermatologic Agents; Immunosuppressive Agents; Radiation-Sensitizing Agents /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health(NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Dimethyl fumarate is included in the database. Tecfidera is indicated for the treatment of patients with relapsing forms of multiple sclerosis. /Included in US product label/ EXPL THER Mixtures of fumaric acid esters (FAE) are used as an oral systemic treatment for moderate to severe psoriasis. Large clinical studies with dimethylfumarate (DMF) monotherapy are scarce. The objective of this study is to assess the effectiveness and long-term safety of high-dose DMF monotherapy in moderate to severe psoriasis. A prospective single-blinded follow-up study was performed in a cohort of patients treated with DMF. Patients were followed-up at fixed intervals. Assessment of consecutive photographs was performed by two observers. Primary outcome was a change in static physician global assessment (PGA) score. Safety outcome was defined as incidences of (serious) adverse events. A total of 176 patients with moderate to severe psoriasis were treated with DMF for a median duration of 28 months. The median daily maintenance dosage of 480 mg was reached after a median of 8 months. Psoriasis activity decreased significantly by 1.7 out of five points. A total of 152 patients reported one or more adverse events, such as gastrointestinal complaints and flushing. High-dose DMF monotherapy is an effective and safe treatment option in moderate to severe psoriasis. It can be suggested that 50% of all patients may benefit from high-dose DMF monotherapy. KEYWORDS: Dimethylfumurate; high dose; monotherapy; prospective study; psoriasis Drug Warnings A patient with multiple sclerosis who was being treated with dimethyl fumarate developed progressive multifocal leukoencephalopathy (PML), and later died. The patient who died was not taking any other drugs that affect the immune system or drugs that are thought to be associated with PML. Patients taking dimethyl fumarate should be advised to contact their clinician if they develop any symptoms that may be suggestive of PML. Symptoms of PML are diverse, progress over days to weeks, and include the following: progressive weakness on one side of the body or clumsiness of limbs; disturbance of vision; and changes in thinking, memory and orientation, leading to confusion and personality changes. The progression of deficits can lead to severe disability or death. Dimethyl fumarate should be discontinued immediately at the first sign or symptom suggestive of PML and an appropriate diagnostic evaluation should be performed. Lymphocyte counts should be monitored in dimethyl fumarate-treated patients according to approved labeling. Dimethyl fumarate may decrease lymphocyte counts. In placebo-controlled clinical trials, mean lymphocyte counts decreased by approximately 30% during the first year of treatment with the drug and remained stable thereafter. Mean lymphocyte counts improved 4 weeks following discontinuance of the drug, but did not return to baseline values. Dimethyl fumarate has not been studied in patients with preexisting low lymphocyte counts. Prior to initiation of dimethyl fumarate, a recent (i.e., within 6 months) complete blood cell (CBC) count should be available to identify patients with preexisting low lymphocyte counts. A CBC should also be obtained annually during therapy and as clinically indicated. In patients with serious infections, withholding dimethyl fumarate treatment should be considered until the infection has resolved. During post marketing experience, hypersensitivity reactions have been reported, including rare reports of anaphylaxis and angioedema in patients treated with Tecfidera. Signs and symptoms have included difficulty breathing, urticaria, and swelling of the throat and tongue. Treatment with Tecfidera should not be initiated in patients with signs and symptoms of a serious infection. Decreases in lymphocyte counts observed in patients treated with Tecfidera in clinical trials were not associated with increased frequencies of infections. However, due to the potential risk of infections in patients who develop sustained lymphopenia, patients should be instructed to report symptoms of infection to their physician. For patients with signs and symptoms of serious infections, interrupting treatment with Tecfidera should be considered, until the infection(s) resolves. For more Drug Warnings (Complete) data for DIMETHYL FUMARATE (14 total), please visit the HSDB record page. Pharmacodynamics The physiological effects of dimethyl fumarate on the body are not well understood. It has anti-inflammatory and cytoprotective effects, likely involved in its actions in multiple sclerosis (MS) patients. Dimethyl fumarate does not cause clinically significant QT interval prolongation. However, cases of progressive multifocal leukoencephalopathy, serious opportunistic infections, lymphopenia and liver injury have been reported in MS patients treated with this drug. Dimethyl fumarate may also cause anaphylaxis and angioedema. |
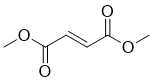
| 分子式 |
C6H8O4
|
|---|---|
| 分子量 |
144.12
|
| 精确质量 |
144.042
|
| 元素分析 |
C, 50.00; H, 5.60; O, 44.40
|
| CAS号 |
624-49-7
|
| 相关CAS号 |
Dimethyl fumarate-d6;66487-95-4;Dimethyl fumarate-d2;23057-98-9
|
| PubChem CID |
637568
|
| 外观&性状 |
White to off-white solid
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
193.0±0.0 °C at 760 mmHg
|
| 熔点 |
102-106 °C(lit.)
|
| 闪点 |
91.1±0.0 °C
|
| 蒸汽压 |
0.5±0.3 mmHg at 25°C
|
| 折射率 |
1.435
|
| 来源 |
Endogenous Metabolite
|
| LogP |
0.62
|
| tPSA |
52.6
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
10
|
| 分子复杂度/Complexity |
141
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O(C([H])([H])[H])C(/C(/[H])=C(\[H])/C(=O)OC([H])([H])[H])=O
|
| InChi Key |
LDCRTTXIJACKKU-ONEGZZNKSA-N
|
| InChi Code |
InChI=1S/C6H8O4/c1-9-5(7)3-4-6(8)10-2/h3-4H,1-2H3/b4-3+
|
| 化学名 |
But-2-enedioic acid dimethyl ester
|
| 别名 |
DMF Dimethylfumarate Dimethyl Fumarate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~41.67 mg/mL (~289.11 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (14.43 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (14.43 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (14.43 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 2 mg/mL (13.88 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶 (<60°C). 配方 5 中的溶解度: 7.5 mg/mL (52.04 mM) in 50% PEG300 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 6.9387 mL | 34.6933 mL | 69.3866 mL | |
| 5 mM | 1.3877 mL | 6.9387 mL | 13.8773 mL | |
| 10 mM | 0.6939 mL | 3.4693 mL | 6.9387 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Dimethyl Fumarate in Adrenomyeloneuropathy
CTID: NCT06513533
Phase: Phase 2/Phase 3 Status: Recruiting
Date: 2024-07-25
|