| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
CFTR/cystic fibrosis transmembrane conductance regulator
|
|---|---|
| 体外研究 (In Vitro) |
为了恢复 Phe508del CFTR 蛋白功能,elexacaftor (VX-445) 是下一代囊性纤维化跨膜调节剂 (CFTR) 校正剂。用 elexacaftor (VX-445) 治疗囊性纤维化是可能的。当串联使用时,VX-445-Tezacaftor-VX-770 极大地增强了 Phe508del CFTR 蛋白的加工、运输和氯离子运输,同时还增加了每种化学物质的数量至其组合的程度 [2]。
在体外实验中,VX-445-特扎卡福-伊伐卡福显著改善了Phe508del CFTR蛋白的加工、运输和氯离子转运,其改善程度大于双重组合中的任何两种药物[2]。 |
| 体内研究 (In Vivo) |
在囊性纤维化患者中,VX-445-特扎卡福-伊伐卡福具有可接受的安全性和副作用。大多数不良事件为轻度或中度。治疗还使Phe508del-MF组的预测FEV1百分比增加了13.8分(P<0.001)。在Phe508del-Phe508del组中已经接受特扎卡福-伊伐卡福治疗的患者中,添加VX-445导致预测FEV1的百分比增加11.0个百分点(P<0.001)。在两组中,汗液氯化物浓度均有所降低,囊性纤维化问卷修订版的呼吸域评分有所改善。
结论:
使用VX-445-tezachaftor-ivacaftor靶向Phe508del CFTR蛋白导致体外CFTR功能增强,并转化为具有一个或两个Phe508del等位基因的囊性纤维化患者的改善。这种方法有可能治疗大约90%的患者囊性纤维化的根本原因[2]。
|
| 酶活实验 |
使用室测定法[2]
使用腔室技术记录了由于CFTR介导的HBE细胞中的氯化物转运引起的短路跨上皮电流。在记录氯化物转运之前,将HBE细胞与化合物一起孵育18至24小时。如前所述,在阿米洛利、叉索林和CFTR抑制剂存在的情况下测量了CFTR介导的氯化物转运。 |
| 细胞实验 |
通过免疫印迹技术评估CFTR加工和贩运。简而言之,用含0.4%碳酸氢钠的HBSS在37°C下洗涤一次HBE细胞3小时,然后用温PBS洗涤两次,然后在37°C下与DMSO、伊伐卡福和特扎卡福、单独的VX-445或VX-445-特扎卡佛或伊伐卡佛的组合在HBE培养基中孵育24小时。孵育后,细胞在含有无EDTA蛋白酶抑制剂的冰冷CHAPS裂解缓冲液中裂解。在4°C下以10000×g的速度将裂解物旋转15分钟,使核和不溶性物质沉淀。将约12μg的总蛋白与含有5%β-巯基乙醇的2x Laemmli样品缓冲液混合,并装载到3%至8%的三乙酸甘油酯凝胶上。将凝胶转移到硝化纤维膜上,并使用1:1000稀释的单克隆CFTR抗体596和1:5000稀释的calnexin兔单克隆抗体进行蛋白质印迹。用于CFTR的第二抗体是1:5000稀释的驴抗小鼠HRP抗体,用于calnexin的是1:5000稀释剂的驴抗兔HRP抗体。印迹由SuperSignal™West Dura延长持续时间基质(Thermo Fisher Scientific,Waltham,MA)开发,然后由myECL成像仪进行可视化。使用Image Studio Lite对带C、带B和calnexin的相对量进行定量。为了量化CFTR成熟度,将CFTR C带蛋白的相对量归一化为在相同蛋白质样本中测量的calnexin,并将这些水平用于后续计算[2]。
|
| 动物实验 |
Clinical Development[2]
After a phase 1 trial involving healthy volunteers (not reported here), a three-part, randomized, double-blind, placebo- or active-controlled, parallelgroup, dose-ranging, phase 2 trial was conducted from July 2017 through March 2018. Patients 18 years of age or older with cystic fibrosis were enrolled at 38 sites in the United States, the Netherlands, Belgium, and Australia. The trial design and conduct were similar to those presented in the companion trial of VX-6591 (see page 7 in the Supplementary Appendix for details). Patients with Phe508del–MF genotypes were randomly assigned to receive 4 weeks of active treatment — with VX-445 at a dose of 50, 100, or 200 mg orally once daily in triple combination with tezacaftor (100 mg per day) and ivacaftor (150 mg every 12 hours) — or a triple placebo control. Patients with the Phe508del–Phe508del genotype received a 4-week run-in with tezacaftor and ivacaftor and were randomly assigned to receive 4 weeks of treatment with either VX-445 (200 mg per day orally) plus tezacaftor (100 mg per day) and ivacaftor (150 mg every 12 hours) or matched placebo plus tezacaftor and ivacaftor. In addition, the trial included patients with Phe508del–MF genotypes treated with VX-445 in triple combination with tezacaftor and VX-561, a deuterated form of ivacaftor taken once daily, or triple placebo. For details regarding trial design and oversight, including a description of VX-561, trial participants, and assessments, see the accompanying article on VX-659 by Davies et al.1 and the Supplementary Appendix of this article (pages 7 through 14, Fig. S1, and Table S1). Clinical Efficacy[2] Clinical efficacy was evaluated on the basis of the change in forced expiratory volume in 1 second (FEV1) from baseline and a disease-specific health-related quality-of-life instrument, the Cystic Fibrosis Questionnaire–Revised (CFQ-R). Each CFQ-R domain is scored on a 100-point scale, with higher scores indicating a lower effect of symptoms on the patient’s quality of life. A minimal clinically important difference of 4 points has been determined for the respiratory symptoms domain. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The absolute oral bioavailability of elexacaftor is approximately 80%. The steady-state AUC0-24h and Cmax following once daily dosing with elexacaftor 200mg are 162 mcg∙h/mL and 8.7 mcg/mL, respectively, and the median Tmax is 6 hours. The AUC of elexacaftor is increased 1.9-2.5-fold following a moderate-fat meal - for this reason, it is recommended to give TrikaftaTM with fat-containing food. Approximately 87.3% of an administered radio-labeled dose of elexacaftor was found in the feces, mostly as metabolites, while only 0.23% of that same dose was found excreted in the urine. The apparent volume of distribution of elexacaftor is 53.7 L. The mean apparent clearance of elexacaftor is 1.18 L/h. Metabolism / Metabolites The metabolism of elexacaftor is extensive and primarily catalyzed via CYP3A4/5. Its main active metabolite, M23-ELX, carries a similar potency as the parent drug. The precise metabolic pathway of elexacaftor has not yet been elucidated in published research. Biological Half-Life The mean terminal half-life of elexacaftor is approximately 24.7 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
Elexacaftor is >99% protein bound in plasma, primarily to albumin. |
| 参考文献 |
|
| 其他信息 |
Elexacaftor (previously VX-445) is a small molecule, next-generation corrector of the cystic fibrosis transmembrane conductance regulator (CFTR) protein. It received FDA approval in October 2019 in combination with [tezacaftor] and [ivacaftor] as the combination product TrikaftaTM. Elexacaftor is considered a next-generation CFTR corrector as it possesses both a different structure and mechanism as compared to first generation correctors like tezacaftor. While dual corrector/potentiator combination therapy has proven useful in the treatment of a subset of CF patients, their use is typically limited to patients who are homozygous for the F508del-CFTR gene. Elexacaftor, along with [VX-659], was designed to fill the need for an efficacious CF therapy for patients who are heterozygous for F508del-CFTR and a gene that does not produce protein or produces proteins unresponsive to ivacaftor or tezacaftor. The triple combination product TrikaftaTM, manufactured by Vertex Pharmaceuticals, is the first product approved for the treatment of CF in individuals who are either homo- or heterozygous for the F508del-CFTR gene - this represents approximately 70-90% of all CF patients.
Drug Indication Elexacaftor, in combination with [ivacaftor] and [tezacaftor] as the combination product TrikaftaTM, is indicated for the treatment of cystic fibrosis (CF) in patients 12 years of age and older who have at least one _F508del_ mutation in the CTFR gene. Mechanism of Action Cystic fibrosis (CF) is the result of a mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. The CFTR proteins produced by this gene are transmembrane ion channels that move sodium and chloride across cell membranes - water follows the flow of chloride ions to the cell surface, which consequently helps to hydrate the surface of the cell and thin the secretions (i.e. mucous) around the cell. Mutations in the CFTR gene produce CFTR proteins of insufficient quantity and/or function, leading to defective ion transport and a build-up of thick mucous throughout the body that causes multi-organ disease involving the pulmonary, gastrointestinal, and pancreatic systems (amongst others). The most common CFTR mutation, the _F508del_ mutation, is estimated to account for 70 to 90% of all CFTR mutations and results in severe processing and trafficking defects of the CFTR protein. Elexacaftor is a CFTR corrector that modulates CFTR proteins to facilitate trafficking to the cell surface for incorporation into the cell membrane. The end result is an increase in the number of mature CFTR proteins present at the cell surface and, therefore, improved ion transport and CF symptomatology. Elexacaftor is used in combination with tezacaftor, another CFTR corrector with a different mechanism of action, and ivacaftor, a CFTR potentiator that improves the function of CFTR proteins on the cell surface - this multi-faceted, triple-drug approach confers a synergistic effect beyond that seen in typical corrector/potentiator dual therapy regimens. |
| 分子式 |
C26H34F3N7O4S
|
|---|---|
| 分子量 |
597.6529
|
| 精确质量 |
597.23
|
| 元素分析 |
C, 52.25 H, 5.73 F, 9.54 N, 16.41 O, 10.71 S, 5.36
|
| CAS号 |
2216712-66-0
|
| 相关CAS号 |
(R)-Elexacaftor;2229860-99-3;Elexacaftor-d3;Elexacaftor-13C,d3
|
| PubChem CID |
134587348
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
4.9
|
| tPSA |
133
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
11
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
41
|
| 分子复杂度/Complexity |
1050
|
| 定义原子立体中心数目 |
1
|
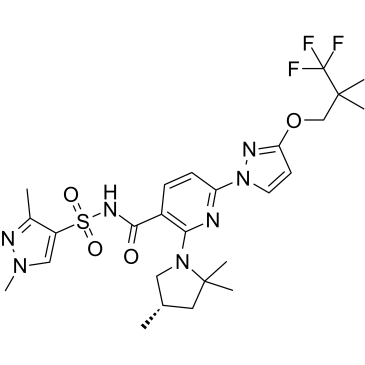
| SMILES |
C[C@H]1CC(N(C1)C2=C(C=CC(=N2)N3C=CC(=N3)OCC(C)(C)C(F)(F)F)C(=O)NS(=O)(=O)C4=CN(N=C4C)C)(C)C
|
| InChi Key |
MVRHVFSOIWFBTE-INIZCTEOSA-N
|
| InChi Code |
InChI=1S/C26H34F3N7O4S/c1-16-12-25(5,6)35(13-16)22-18(23(37)33-41(38,39)19-14-34(7)31-17(19)2)8-9-20(30-22)36-11-10-21(32-36)40-15-24(3,4)26(27,28)29/h8-11,14,16H,12-13,15H2,1-7H3,(H,33,37)/t16-/m0/s1
|
| 化学名 |
(S)-N-((1,3-dimethyl-1H-pyrazol-4-yl)sulfonyl)-6-(3-(3,3,3-trifluoro-2,2-dimethylpropoxy)-1H-pyrazol-1-yl)-2-(2,2,4-trimethylpyrrolidin-1-yl)nicotinamide
|
| 别名 |
Elexacaftor, WHO 11180; WHO 11180; RRN67GMB0V; UNII-RRN67GMB0V; Elexacaftor (USAN); ELEXACAFTOR [MI]; WHO11180; VX-445; VX 445; VX445; Trikafta
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~125 mg/mL (~209.15 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (3.48 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (3.48 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6732 mL | 8.3661 mL | 16.7322 mL | |
| 5 mM | 0.3346 mL | 1.6732 mL | 3.3464 mL | |
| 10 mM | 0.1673 mL | 0.8366 mL | 1.6732 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT06331000 | Not yet recruiting | Drug: elexacaftor-tezacaftor-ivacaftor treatment |
Cystic Fibrosis | University Hospital, Strasbourg, France | March 2024 | |
| NCT05576324 | Recruiting | Drug: Elexacaftor / Ivacaftor / Tezacaftor | Cystic Fibrosis | University of Erlangen-Nürnberg Medical School |
December 30, 2020 | |
| NCT06184763 | Active, not recruiting | Other: 6-minute walk test | Cystic Fibrosis | Hospices Civils de Lyon | August 1, 2023 | |
| NCT06072365 | Completed | Other: nutritional intake questionnaire |
Cystic Fibrosis | University Hospital, Toulouse | October 21, 2021 |