| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|

| 靶点 |
AR/androgen-receptor (IC50 = 36 nM in LNCaP cells)[1]
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
在使用 16β-[18F]氟-5α-DHT (18-FDHT) 的竞争性测定中,去势抵抗性 LNCaP/AR 细胞中的恩杂鲁胺 (MDV3100) 对 AR 的亲和力高于 ICI 176334(AR 过表达)。对 LNCaP/AR 前列腺细胞,依折麦布没有激动作用。 Enzalutamide 通过与亲代 LNCaP 细胞中存在的合成雄激素 R1881 结合,抑制跨膜丝氨酸蛋白酶 2 (TMPRSS2) 和前列腺特异性抗原 (PSA) 的激活。突变 AR 蛋白(W741C,Trp741 改为 Cys)的转录活性受到 enzalutamide 的抑制 [1]。此外,恩杂鲁胺抑制共激活剂的募集和配体-受体复合物的核转位[2]。
|
||
| 体内研究 (In Vivo) |
当以 10 mg/kg 的剂量给予携带 LNCaP/AR 异种移植物的去势雄性小鼠时,恩杂鲁胺 (MDV3100) 显着减少肿瘤生长 [1]。当以 0.5 至 5 mg/kg 的剂量口服给药时,恩杂鲁胺的药代动力学表现出剂量依赖性[4]。
MDV3100/enzalutamide和RD162在去势抵抗的人前列腺癌小鼠模型中可诱导肿瘤消退。在首批接受MDV3100治疗的I/II期临床试验中,30名患者中有13名(43%)的血清前列腺特异性抗原(前列腺癌的生物标志物)浓度持续下降(>50%)。因此,这些化合物似乎是治疗晚期前列腺癌的有希望的候选者。[1] 恩杂鲁胺治疗可降低小鼠LNCaP-AR异种移植瘤体积、增加体重并诱导细胞凋亡[2] 为了研究恩杂鲁胺和比卡鲁胺在体内的作用,我们用阉割的雄性动物植入过表达野生型AR 25的人LNCaP-AR细胞,建立小鼠异种移植CRPC模型。小鼠给予恩杂鲁胺(1 ~ 50 mg/kg/d)或比卡鲁胺(50 mg/kg/d),每隔2 ~ 3天测量肿瘤体积和小鼠体重,连续28天。比卡鲁胺(50 mg/kg/天)与对照组相比,抑制肿瘤生长至第16天。然而,在第16天之后,这些小鼠的肿瘤持续生长,到第28天达到基线的154%(图3A,表1)。相比之下,在治疗的前6天,与对照药和比卡鲁胺治疗的小鼠相比,恩杂鲁胺(10 mg/kg/天)显著抑制肿瘤生长(相对于基线的肿瘤生长平均±SE百分比:对照药,119±5%;恩杂鲁胺10 mg/kg, 86±6%,比卡鲁胺50 mg/kg, 106±8%)。到第13天,在10 mg/kg/天或更高剂量的enzalutamide治疗下,与初始肿瘤大小相比,肿瘤体积减少了19%(图3A)。在恩扎鲁胺处理组(1和50 mg/kg)中,一些肿瘤的大小明显减小,超出了测量极限(表1,不可测量的肿瘤/组)。这些肿瘤未包括在进一步的分析中。10 mg/kg/天的恩杂鲁胺组在第24天肿瘤体积继续减小,50 mg/kg/天的恩杂鲁胺组在第28天的最后一个测量时间点(表1)。相对于初始肿瘤体积,各组肿瘤消退的最大效果出现在恩杂鲁胺治疗的第28天或之后(图3A)。enzalutamide治疗27天后,与基线相比,10 mg/kg时平均±SE相对肿瘤体积下降41±7%,50 mg/kg时平均±SE相对肿瘤体积下降68±13%。相比之下,经过27天的对照或50 mg/kg比卡鲁胺治疗后,肿瘤体积比基线增加了54%(表1)。 药代动力学分析[4] 采用线性梯形法计算等离子体浓度-时间曲线(AUC)和第一矩曲线(AUMC)下的面积,外推至时间=无穷大。终端半衰期(T½)计算为0.693/λ,其中λ为浓度-时间曲线的对数线性部分的斜率。系统清除率(CL)、平均停留时间(MRT)和稳态分布体积(Vss)分别用剂量/AUC、AUMC/AUC和MRT·CL计算。绝对口服生物利用度(F)通过口服给药后的AUC除以相应剂量静脉给药后的AUC来估计。峰值浓度(Cmax)和达到Cmax的时间(Tmax)直接从个体血浆浓度-时间曲线中读取。用给药后平均AUCtissue除以平均AUCplasma计算enzalutamide的组织-血浆分配系数(Kp)。为了获得上述药代动力学参数,使用WinNonlin软件,采用非线性最小二乘回归的非区室方法分析所有血浆和组织浓度-时间曲线。 |
||
| 酶活实验 |
大鼠肝微粒体对恩杂鲁胺肝内清除率的测定[4]
用大鼠肝微粒体测定对恩杂鲁胺的内在清除率。典型反应混合物(500µL)由大鼠肝微粒体蛋白(终浓度= 0.5 mg蛋白/mL孵育混合物)和NADPH再生体系(终浓度:1.3 mM NADP+、3.3 mM葡萄糖-6-磷酸、0.4 U/mL葡萄糖-6-磷酸脱氢酶和3.3 mM氯化镁)在100 mM磷酸钾缓冲液(pH 7.4)中组成。将混合物在37℃水浴中预孵育5分钟,加入一等分恩杂鲁胺溶液至终浓度为2µM。在反应开始后的0、5、15和30分钟取样等分(50µL)的混合物。收集后,立即向样品中加入停止溶液(100µL冰冻甲醇)以终止反应。剧烈涡流后,在10,000×g下离心5分钟,取50µL的上清液进行测定。将样品中剩余的enzalutamide浓度与反应时间进行对比,以确定反应的代谢速率常数。 enzalutamide与血浆蛋白结合比例的估算[4] 我们进行了蛋白结合研究,以确定未结合的恩杂鲁胺在大鼠血浆中的比例。使用RED®设备通过平衡透析评估测试材料的结合。所有摊款一式三份。将含有2µg/mL enzalutamide的200µL血浆样品放入样品室后,在缓冲室中加入350µL磷酸盐缓冲液(pH 7.4)。将装有样品的装置在37℃的摇水浴中孵育4小时。孵育后,检测血浆和缓冲液中恩杂鲁胺的含量。 |
||
| 细胞实验 |
核易位试验[3]
黄色荧光蛋白(YFP)-AR质粒由Marc I. Diamond捐赠,稳定转染到HEK293细胞中。将细胞以1.5 × 105个/cm2的速度接种于无酚红的DMEM/F12培养基中,并添加10%去激素FBS。培养2天后,用恩杂鲁胺(1µM)或比卡鲁胺(1µM)预处理细胞2小时,然后在恩杂鲁胺或比卡鲁胺存在下,用1 nM DHT共处理1小时。细胞用磷酸盐缓冲盐水洗涤,用核荧光标记物DAPI(1µg/ml)孵育30分钟,室温下用4%多聚甲醛固定30分钟。使用Qimaging数码相机与使用YFP滤光片的Olympus X71荧光显微镜相连接,对细胞进行可视化。使用ImageJ软件定量细胞核和总细胞AR-YFP荧光强度(积分密度)。在基于DAPI荧光的图像分割定义的区域内定量核AR-YFP荧光,并计算核:总强度比。每个独立实验每个条件至少定量14个细胞(n = 3)。对于活细胞成像实验,细胞用恩杂鲁胺(1或10µM)或比卡鲁胺(1或10µM)预处理2小时,然后在恩杂鲁胺或比卡鲁胺存在下用1 nM DHT共处理3小时。在加入DHT (t′= 0)之前立即对细胞进行成像,然后在3小时内间隔60分钟对细胞进行成像。 |
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The median Tmax is 1 hour (0.5 to 3 hours) following a single 160 mg dose of capsules and 2 hours (0.5 to 6 hours) following a single 160 mg dose of tablets. Enzalutamide achieves steady-state by Day 28 and its AUC accumulates approximately 8.3-fold relative to a single dose. At steady-state, the mean (%CV) maximum concentration (Cmax) for enzalutamide and N-desmethyl enzalutamide is 16.6 µg/mL (23%) and 12.7 µg/mL (30%), respectively, and the mean (%CV) minimum concentrations (Cmin) are 11.4 µg/mL (26%) and 13.0 µg/mL (30%), respectively. Enzalutamide is primarily eliminated by hepatic metabolism. 71% of the dose is recovered in urine (including only trace amounts of enzalutamide and N-desmethyl enzalutamide), and 14% is recovered in feces (0.4% of the dose as unchanged enzalutamide and 1% as N-desmethyl enzalutamide). The mean (%CV) volume of distribution after a single oral dose is 110 L (29%). The mean apparent clearance (CL/F) of enzalutamide after a single dose is 0.56 L/h (0.33 to 1.02 L/h). Metabolism / Metabolites Enzalutamide is metabolized by CYP2C8 and CYP3A4. CYP2C8 is primarily responsible for the formation of the active metabolite (N-desmethyl enzalutamide). Carboxylesterase 1 metabolizes N-desmethyl enzalutamide and enzalutamide to the inactive carboxylic acid metabolite. Biological Half-Life The mean terminal half-life (t1/2) for enzalutamide in patients after a single oral dose is 5.8 days (range 2.8 to 10.2 days). Following a single 160 mg oral dose of enzalutamide in healthy volunteers, the mean terminal t1/2 for N-desmethyl enzalutamide is approximately 7.8 to 8.6 days. Researchers characterized the pharmacokinetics of enzalutamide, a novel anti-prostate cancer drug, in rats after intravenous and oral administration in the dose range 0.5-5 mg/kg. Tissue distribution, liver microsomal stability, and plasma protein binding were also examined. After intravenous injection, systemic clearance, volumes of distribution at steady state (Vss), and half-life (T½) remained unaltered as a function of dose, with values in the ranges of 80.4-86.3 mL/h/kg, 1020-1250 mL/kg, and 9.13-10.6 h, respectively. Following oral administration, absolute oral bioavailability was 89.7 % and not dose-dependent. The recoveries of enzalutamide in urine and feces were 0.0620 and 2.04 %, respectively. Enzalutamide was distributed primarily in 10 tissues (brain, liver, kidneys, testis, heart, spleen, lungs, gut, muscle, and adipose) and tissue-to-plasma ratios of enzalutamide ranged from 0.406 (brain) to 10.2 (adipose tissue). Further, enzalutamide was stable in rat liver microsomes, and its plasma protein binding was 94.7 %. In conclusion, enzalutamide showed dose-independent pharmacokinetics at intravenous and oral doses of 0.5-5 mg/kg. Enzalutamide distributed primarily to 10 tissues and appeared to be eliminated primarily by metabolism. [4] |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In preregistration controlled trials, serum aminotransferase elevations occurred in up to 10% patients treated with enzalutamide, but similar somewhat high rates occurred in patients receiving placebo (~9%). The liver test abnormalities were generally mild, transient and not associated with symptoms or jaundice. ALT elevations above 5 times the ULN were rare (0.2%) and also no more frequent than with placebo therapy. In addition, clinically apparent liver injury with jaundice was not reported in the preregistration trials of enzalutamide, and clinically apparent liver injury and hepatitis are not mentioned in the product label. Since the approval and more wide scale use of enzalutamide, there have been no publications or descriptions of the clinical features of hepatotoxicity with jaundice associated with its use. Thus, clinically apparent liver injury due to enzalutamide must be rare, if it occurs at all. Likelihood score: E (unlikely cause of clinically apparent liver injury). Protein Binding Enzalutamide is 97% to 98% bound to plasma proteins, primarily albumin. N-desmethyl enzalutamide is 95% bound to plasma proteins. |
||
| 参考文献 |
|
||
| 其他信息 |
Pharmacodynamics
Enzalutamide is a second-generation antiandrogen that blocks the activity of androgen and androgen receptor (AR) in prostate cancer. AR activity and prostate cancer progression are closely related due to the normal physiology of prostate cells, providing the rationale for androgen deprivation therapy (ADT). However, resistance will eventually arise after the commencement of ADT in 2-3 years due to the accumulation of mutations, including constitutively active mutation, AR overexpression, and changes in AR splicing variants. Enzalutamide was therefore designed to exploit these mutations. In vitro experiments in human prostate cancer cell line VCaP showed that enzalutamide can suppress cell growth and induce apoptosis while other antiandrogens like bicalutamide did not. Clinical trials on prostate cancer patients indicated that enzalutamide can lead to a decrease in serum PSA for at least 12 weeks, although this response can be short-lived and thus resulting in enzalutamide resistance. Patients receiving enzalutamide also had a 37% decreased in the risk of death compared to placebo. Metastatic prostate cancer is treated with drugs that antagonize androgen action, but most patients progress to a more aggressive form of the disease called castration-resistant prostate cancer, driven by elevated expression of the androgen receptor. Here we characterize the diarylthiohydantoins RD162 and MDV3100, two compounds optimized from a screen for nonsteroidal antiandrogens that retain activity in the setting of increased androgen receptor expression. Both compounds bind to the androgen receptor with greater relative affinity than the clinically used antiandrogen bicalutamide, reduce the efficiency of its nuclear translocation, and impair both DNA binding to androgen response elements and recruitment of coactivators. RD162 and MDV3100 are orally available and induce tumor regression in mouse models of castration-resistant human prostate cancer. Of the first 30 patients treated with MDV3100 in a Phase I/II clinical trial, 13 of 30 (43%) showed sustained declines (by >50%) in serum concentrations of prostate-specific antigen, a biomarker of prostate cancer. These compounds thus appear to be promising candidates for treatment of advanced prostate cancer.[1] Background: MDV3100 is an androgen-receptor antagonist that blocks androgens from binding to the androgen receptor and prevents nuclear translocation and co-activator recruitment of the ligand-receptor complex. It also induces tumour cell apoptosis, and has no agonist activity. Because growth of castration-resistant prostate cancer is dependent on continued androgen-receptor signalling, we assessed the antitumour activity and safety of MDV3100 in men with this disease. Methods: This phase 1-2 study was undertaken in five US centres in 140 patients. Patients with progressive, metastatic, castration-resistant prostate cancer were enrolled in dose-escalation cohorts of three to six patients and given an oral daily starting dose of MDV3100 30 mg. The final daily doses studied were 30 mg (n=3), 60 mg (27), 150 mg (28), 240 mg (29), 360 mg (28), 480 mg (22), and 600 mg (3). The primary objective was to identify the safety and tolerability profile of MDV3100 and to establish the maximum tolerated dose. The trial is registered with ClinicalTrials.gov, number NCT00510718. Findings: We noted antitumour effects at all doses, including decreases in serum prostate-specific antigen of 50% or more in 78 (56%) patients, responses in soft tissue in 13 (22%) of 59 patients, stabilised bone disease in 61 (56%) of 109 patients, and conversion from unfavourable to favourable circulating tumour cell counts in 25 (49%) of the 51 patients. PET imaging of 22 patients to assess androgen-receptor blockade showed decreased (18)F-fluoro-5alpha-dihydrotestosterone binding at doses from 60 mg to 480 mg per day (range 20-100%). The median time to progression was 47 weeks (95% CI 34-not reached) for radiological progression. The maximum tolerated dose for sustained treatment (>28 days) was 240 mg. The most common grade 3-4 adverse event was dose-dependent fatigue (16 [11%] patients), which generally resolved after dose reduction. Interpretation: We recorded encouraging antitumour activity with MDV3100 in patients with castration-resistant prostate cancer. The results of this phase 1-2 trial validate in man preclinical studies implicating sustained androgen-receptor signalling as a driver in this disease. [2] Background: Enzalutamide (formerly MDV3100 and available commercially as Xtandi), a novel androgen receptor (AR) signaling inhibitor, blocks the growth of castration-resistant prostate cancer (CRPC) in cellular model systems and was shown in a clinical study to increase survival in patients with metastatic CRPC. Enzalutamide inhibits multiple steps of AR signaling: binding of androgens to AR, AR nuclear translocation, and association of AR with DNA. Here, we investigate the effects of enzalutamide on AR signaling, AR-dependent gene expression and cell apoptosis. Methods: The expression of AR target gene prostate-specific antigen (PSA) was measured in LnCaP and C4-2 cells. AR nuclear translocation was assessed in HEK-293 cells stably transfected with AR-yellow fluorescent protein. The in vivo effects of enzalutamide were determined in a mouse xenograft model of CRPC. Differential gene expression in LNCaP cells was measured using Affymetrix human genome microarray technology. Results: We found that unlike bicalutamide, enzalutamide lacked AR agonistic activity at effective doses and did not induce PSA expression or AR nuclear translocation. Additionally, it is more effective than bicalutamide at inhibiting agonist-induced AR nuclear translocation. Enzalutamide induced the regression of tumor volume in a CRPC xenograft model and apoptosis in AR-over-expressing prostate cancer cells. Finally, gene expression profiling in LNCaP cells indicated that enzalutamide opposes agonist-induced changes in genes involved in processes such as cell adhesion, angiogenesis, and apoptosis. Conclusions: These results indicate that enzalutamide efficiently inhibits AR signaling, and we suggest that its lack of AR agonist activity may be important for these effects.[3] |
| 分子式 |
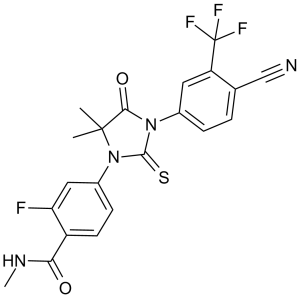
C21H16F4N4O2S
|
|---|---|
| 分子量 |
464.44
|
| 精确质量 |
464.093
|
| 元素分析 |
C, 54.31; H, 3.47; F, 16.36; N, 12.06; O, 6.89; S, 6.90
|
| CAS号 |
915087-33-1
|
| 相关CAS号 |
N-desmethyl Enzalutamide;1242137-16-1;N-desmethyl Enzalutamide-d6;Enzalutamide carboxylic acid;1242137-15-0;Deutenzalutamide-d3;1443331-82-5;Enzalutamide-d6;1443331-94-9
|
| PubChem CID |
15951529
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 折射率 |
1.630
|
| LogP |
2.13
|
| tPSA |
108.53
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
32
|
| 分子复杂度/Complexity |
839
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
WXCXUHSOUPDCQV-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C21H16F4N4O2S/c1-20(2)18(31)28(12-5-4-11(10-26)15(8-12)21(23,24)25)19(32)29(20)13-6-7-14(16(22)9-13)17(30)27-3/h4-9H,1-3H3,(H,27,30)
|
| 化学名 |
4-[3-[4-cyano-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-sulfanylideneimidazolidin-1-yl]-2-fluoro-N-methylbenzamide
|
| 别名 |
MDV-3100; MDV3100; MDV 3100; MDV3100; 4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxoimidazolidin-1-yl)-2-fluoro-N-methylbenzamide; Enzalutamide (MDV3100); XTANDI; trade name: Xtandi.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.38 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.38 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: 2.5 mg/mL (5.38 mM) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 配方 4 中的溶解度: 15% DMSO +85% PEG 300 : 10mg/mL 配方 5 中的溶解度: 10 mg/mL (21.53 mM) in 1% Tween-80 in PBS (这些助溶剂从左到右依次添加,逐一添加), 悬浮液; 超声助溶 (<60°C). 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1531 mL | 10.7657 mL | 21.5313 mL | |
| 5 mM | 0.4306 mL | 2.1531 mL | 4.3063 mL | |
| 10 mM | 0.2153 mL | 1.0766 mL | 2.1531 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|---|
|
|