| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
P2X7 receptor
|
|---|---|
| 体外研究 (In Vitro) |
Eperisone对成纤维细胞活性的优先抑制作用[1]
我们筛选了临床常用药物库,旨在发现对肺泡上皮细胞无毒但对肺成纤维细胞具有选择性毒性的药物。具体而言,用不同药物处理LL29或A549细胞24小时后,采用甲基噻唑基四唑试剂检测细胞存活率。在LL29细胞IC50值低于A549细胞的药物中,基于两种细胞类型的IC50差异、临床安全性及其他药理活性,我们筛选出艾地苯醌和Eperisone。如前期报道,我们已证实艾地苯醌能优先抑制成纤维细胞活性并缓解博来霉素诱导的肺纤维化。因此本研究聚焦临床用作中枢性肌松剂的Eperisone,通过体外和体内系统评估其对特发性肺纤维化(IPF)的疗效。 如图1A所示,Eperisone处理(25-200μM)可剂量依赖性降低LL29细胞存活率。而200μM Eperisone处理的A549细胞存活率仍达88.5±3.0%(均值±标准误,n=4),表明几乎不影响上皮细胞活性。此外,Eperisone对其他成纤维细胞(HFL-1和IMR-90)同样呈现剂量依赖性增殖抑制(补充图S1A)。在RL-34细胞(大鼠肝源正常上皮细胞)中几乎无毒性,但对可分化为肌成纤维细胞的RI-T细胞(大鼠肝星状细胞)具有优先抑制作用(补充图S1B)。我们进一步采用可检测细胞膜损伤的CellTox™绿色染料分析Eperisone对LL29的细胞毒性。如图1B所示,Eperisone处理呈现时间和浓度依赖性的细胞毒效应。为评估Eperisone对TGF-β1诱导的成纤维细胞活化影响,我们先用Eperisone(10-30μM)预处理LL29细胞,再添加TGF-β1(5μM),72小时后通过实时定量PCR分析纤维化相关因子表达。如图1C所示,TGF-β1可上调LL29细胞中I型胶原(COL1A1)、α-SMA(ACTA2)、结缔组织生长因子(CTGF)、血管内皮生长因子(VEGF)、碱性成纤维细胞生长因子(bFGF)和血小板衍生生长因子(PDGF-A)的mRNA表达,而这些上调均被Eperisone预处理所抑制。这些结果提示Eperisone在体外能优先抑制肺成纤维细胞活性。 其他药物对肺成纤维细胞存活率的影响[1] 如前言所述,吡非尼酮和尼达尼布是目前临床用于治疗IPF的抗纤维化药物。为明确Eperisone对肺成纤维细胞的特异性作用,我们比较了现有药物对LL29和A549细胞存活率的影响。吡非尼酮处理(浓度高达2mM)对两种细胞存活率均无显著影响;尼达尼布虽能降低两种细胞存活率,但抑制程度无细胞类型差异性(图2A)。 Eperisone作为中枢性肌松剂,临床用于改善腰痛和脑血管疾病所致痉挛性瘫痪患者的肌张力。因此我们检测了其他中枢性肌松剂是否具有成纤维细胞选择性。在六种测试药物中,甲苯哌丙酮、伊那哌酮和兰哌酮与Eperisone相似,可优先降低LL29细胞活力;而替扎尼定、美索巴莫和巴氯芬在浓度高达2mM时对两种细胞均无抑制作用(图2B)。如后文将讨论的,由于部分中枢性肌松剂未显示成纤维细胞选择性抑制,我们推测Eperisone可能通过独立于肌松作用之外的分子机制发挥其选择性效应。 盐酸依哌立松于1983年在日本上市,已被用于改善肌肉张力和治疗痉挛性瘫痪。然而,其生化作用机制尚不清楚。SB药物发现用于使用荧光评估嘌呤能P2X(P2X)受体拮抗作用。在这项研究中,我们发现其靶蛋白是P2X7受体。此外,P2X受体亚型选择性很高。这一发现证明了(Eperison-P2X7-pain连锁)、P2X7作为药物靶点的有效性以及盐酸Eperisone药物重新定位的可能性[5]。 |
| 体内研究 (In Vivo) |
Eperisone对博来霉素(BLM)诱导肺纤维化的影响[1]
通过气管内给予BLM诱导雄性ICR小鼠肺纤维化模型。具体而言,在BLM给药10天后,根据体重变化率(排除溶剂对照组)将小鼠分为三组,考察口服Eperisone对肺纤维化的影响。BLM给药20天后制备肺组织切片,采用Masson三色染色法检测胶原沉积。结果显示肺组织胶原沉积呈现BLM剂量依赖性增加,而口服Eperisone则能剂量依赖性抑制这种BLM诱导的胶原沉积(图3A,B)。 随后我们对肺组织中胶原特异性氨基酸——羟脯氨酸进行定量分析。如图3C所示,BLM处理显著增加肺组织羟脯氨酸含量,而Eperisone处理可抑制这一增加。考虑到Eperisone治疗肺纤维化的临床应用价值,除组织学和生化指标外,改善呼吸功能同样至关重要。我们前期研究已证实BLM诱导的肺纤维化会导致肺弹性增加和用力肺活量(FVC)下降。因此本研究采用计算机控制呼吸机和负压储气装置检测小鼠呼吸功能。如图3D所示,BLM处理使总弹性(包括支气管、细支气管和肺泡的整个肺部弹性)和组织弹性(肺泡弹性)显著增加,并降低FVC;而Eperisone能显著改善BLM引起的呼吸功能恶化。这些结果表明Eperisone对BLM诱导的肺纤维化具有改善作用。 目的:Eperisone是一种口服肌肉松弛剂,用于治疗引起肌肉痉挛和疼痛的肌肉骨骼疾病。为了更有效地控制疼痛,依匹立松通常与非甾体抗炎药(NSAIDs)一起服用。因此,与非甾体抗炎药相比,乙哌立松可能被忽视为过敏反应的原因。本研究旨在分析韩国报告的药物不良反应(ADR),并为乙哌立松引起的过敏反应提出适当的诊断方法。 方法:我们回顾了2010年至2015年韩国报告的与Eperisone相关的电子药物警戒数据(韩国药品安全研究所韩国不良事件报告系统[KIDS-KAERS])。选择具有因果关系的ADR。分析了临床表现、严重程度、结果和再暴露信息。为了进一步调查,还审查了单个中心报告的7年ADR数据。该中心进行了口服激发试验(OPT)、皮肤点刺试验(SPT)和嗜碱性粒细胞活化试验(BAT)。 结果:在研究期间,207名患者对依哌立松有不良反应。最常见的不良反应是皮肤过敏反应(30.4%),如荨麻疹、瘙痒或血管性水肿。第五种常见的不良反应是过敏反应。有35名患者发生过敏反应,占依哌立松相关不良反应的16.9%。在单中心研究中,有11名患者发生了依哌立松引起的过敏反应。所有患者均接受了OPT,所有诱发患者均显示阳性反应。11名过敏反应患者中有4名也接受了SPT和BAT检查,结果均为阴性。 结论:根据KIDS-KAERS数据库计算的Eperisone诱导的过敏反应发生率为0.001%。依哌立松可引起超敏反应,包括过敏反应,可能是通过诱导非免疫球蛋白介导的立即超敏反应。 |
| 细胞实验 |
P2X Panel 筛选方案:将稳定表达P2X受体的细胞(P2X1受体为132N1星形细胞瘤细胞,其他P2X受体为HEK293细胞)以每孔50000个细胞的密度接种在黑色透明底96孔板中,并在37°C下孵育过夜。第二天,从细胞板中取出培养基,加入25µL的测定缓冲液(1.11 mM CaCl2、0.43 mM MgCl.6H2O、0.36 mM MgSO4.7H2O、4.98 mM KCl、0.39 mM KH2PO4、122 mM NaCl、0.3 mM Na2HPO4、4.86 mM D-葡萄糖、17.7 mM N-(2-羟乙基)哌嗪-N′-2-乙磺酸(HEPES),pH 7.4)。将钙染料溶液(10µL)加入孔中,在37°C下孵育1小时。加入试验化合物(5µL),在室温下孵育10分钟。然后将平板置于FLIPR中,每1.52秒监测一次荧光。20秒后,加入10µL约EC80浓度的激动剂,在488 nm/510-570 nm的ex/em下监测荧光5分钟。使用GraphPad Prism软件测定测试化合物的IC50值。YO-PRO-1摄取测定方案:将THP-1细胞以每皿10000000个细胞的密度接种到100mm培养皿上,用500 nmol/L佛波醇12-肉豆蔻酸13-乙酸酯处理3小时。收集细胞,用磷酸缓冲盐水(PBS)离心洗涤,并将细胞重新悬浮在培养基(10%胎牛血清(FBS)/RPMI1640)中。将细胞以80000个细胞/0.2 mL/孔的密度接种在黑壁96孔板中,并在37°C下孵育过夜。然后,在孔中加入脂多糖(LPS)(1µg/mL),并将细胞处理6小时(预处理)。用测试化合物处理细胞,将Yo-PRO-1(2µmol/L)加入孔中,在37°C下孵育15分钟。最后,在孔中加入BzATP(300微摩尔/升,P2X7受体激动剂),在ex/em:485nm/535nm下每1分钟监测一次荧光,持续60分钟。测量了10分钟的最大荧光斜率[5]。
|
| 动物实验 |
Treatment of mice with BLM, Eperisone, and other reagents [1]
Mice were anesthetized with isoflurane and intratracheally administered BLM (1 mg/kg, once) in sterile saline via a single channel pipette (P200). Ten days after BLM administration, Eperisone (15 or 50 mg/kg), tolperisone (15 mg/kg), pirfenidone (200 mg/kg), and nintedanib (30 mg/kg) were administered orally for a total of 9 days from day 10 to day 18. Various analyses were then performed on day 20. In the adverse effect study, 10 days after BLM administration, 250 mg/kg of Eperisone was orally administered once, which was five times the dose that showed efficacy. Twenty-four hours after eperisone administration, the fecal condition of the mice was visually examined. In addition, plasma samples and stomach and colon tissues were collected from the mice. Mice were treated with bleomycin (BLM, 1 mg/kg) or vehicle once only on day 0. The mice were then orally administered 250 mg/kg of eperisone (Epe) once at day 10. After 24 h, whole blood was collected from the mice. Analysis of the blood samples was performed by TRANS GENIC INC. Values represent the mean ± SEM.[1] Mice were treated with bleomycin (BLM, 1 mg/kg) or vehicle once only on day 0. The mice were then orally administered 250 mg/kg of eperisone (Epe) once at day 10. After 24 h, the fecal condition (diarrhea or hemorrhagic stool) of the mice was visually examined. The analysis of fecal condition was conducted by an investigator blinded to the study protocol. Gastric mucosal injury and colonic mucosal injury were analyzed based on the hematotoxin and eosin staining images shown in Fig. 5.[1] Mice were treated with bleomycin (BLM, 1 mg/kg) or vehicle once only on day 0. The mice were then orally administered 250 mg/kg of eperisone (Epe) once at day 10. After 24 h, the stomach and colon were collected from the mice. Gastric (A) and colonic (B) tissue sections were prepared and subjected to histopathological examination (hematotoxin and eosin staining; scale bar = 200 µm).[1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Safety analysis of Eperisone administration [1]
In clinical practice, existing IPF treatments, such as pirfenidone and nintedanib, have been reported to induce adverse effects such as increasing markers of liver damage in the plasma and gastrointestinal disorders. Therefore, we conducted a comprehensive analysis of markers for pancreatic, hepatic, and renal damage in plasma. The dose of eperisone was five times higher than the dose that showed efficacy for BLM-dependent pulmonary fibrosis. As shown in Table 1, administration of BLM or BLM plus Eperisone (250 mg/kg, once at day 10) did not significantly alter 12 plasma markers for pancreatic, hepatic, and renal damage. Moreover, administration of eperisone (50 mg/kg) for 9 consecutive days (from day 10 to day 18) did not cause any significant changes in the four plasma markers indicating hepatic and renal damage (Supplementary Table S1). In addition, no mouse exhibited diarrhea or hemorrhagic stool in either group (Table 2). Furthermore, we also examined gastric and colonic mucosal injury using hematoxylin and eosin staining. As shown in Fig. 5 and Table 2, the condition of the gastric and colonic mucosa in mice treated with BLM or BLM plus eperisone (250 mg/kg) was unchanged compared with that in vehicle-treated mice, and no gastric and colonic mucosal injury was observed. These results suggest that eperisone may be able to suppress pulmonary fibrosis without inducing adverse effects. Eperisone hydrochloride (4'-ethyl-2-methyl-3-piperidinopropiophenone hydrochloride) is an antispastic agent used for treatment of diseases characterized by muscle stiffness and pain. The aim of this research was to investigate the efficacy of eperisone in patients with acute low back pain and spasticity of spinal muscles. The study design was a randomized, double-blind (double-dummy) study in 160 patients with low back pain and no Rx finding of major spinal diseases, randomly assigned to a treatment with oral eperisone 100 mg three times daily (t.i.d.) or thiocolchicoside 8 mg twice daily (b.i.d.) for 12 consecutive days. Analgesic activity was evaluated by scoring "spontaneous pain" (VAS) and pain on movement and pression (4-digit scale), while muscle relaxant activity of the medication was evaluated by means of the "hand-to-floor" distance and the Lasegue's manoeuvre. All the measures were done at the inclusion day and after 3, 7 and 12 days of treatment. The two medications had comparable analgesic and muscle relaxant efficacy. Sponta-neous pain and pain on movement/pressure were significantly reduced by both treatments. Moreover, both eperisone- and thiocolchicoside-treated patients showed a clinically evident muscle relaxation as proved by a progressive reduction in the "hand-to-floor" distance and increase in the articular excursion (Lasegue's manoeuvre). Only 5% of eperisone-treated patients showed minor gastrointestinal side effects, while the incidence of side effects in the thiocolchicoside group was 21.25%. Moreover, in the thiocolchicoside-treated patients also diarrhoea was present, which reached a moderate intensity in some cases. In conclusions, eperisone represents a valuable and safer alternative to other muscle relaxant agents for treatment of low back pain. [2] Eperisone, an analgesic and centrally acting muscle relaxant has been in use for the treatment of low back pain (LBP). The present systematic review evaluates the efficacy and safety of eperisone in patients with LBP. Cochrane Back and Neck (CBN) Group and Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines were adopted to perform this systematic review. For risk of bias assessment CBN Group and Moga tools were used. Seven (5 randomized controlled trials [RCTs] and 2 uncontrolled studies) studies involving 801 participants were included. Eperisone intervention may be effective in acute LBP patients with less adverse effects (relative risk, 0.25; 95% confidence interval, 0.15-0.41; p<0.0001). Eperisone also improved paraspinal blood flow and was found to have efficacy similar to tizanidine in chronic LBP patients. The included studies in this review are of smaller sample size and short duration to support eperisone use in LBP. However, we recommend well-designed RCTs of high quality with larger sample size and longer follow-up to confirm the clinical benefits of eperisone in the treatment of acute or chronic LBP. [3] |
| 参考文献 |
[1]. Therapeutic effects of eperisone on pulmonary fibrosis via preferential suppression of fibroblast activity. Cell Death Discov. 2022 Feb 8;8(1):52.
[2]. Efficacy and safety of eperisone in patients with low back pain: a double blind randomized study. Eur Rev Med Pharmacol Sci. 2008 Jul-Aug;12(4):229-35. [3]. Clinical efficacy and safety of eperisone for low back pain: A systematic literature review. Pharmacol Rep. 2016 Oct;68(5):903-12. [4]. Eperisone-Induced Anaphylaxis: Pharmacovigilance Data and Results of Allergy Testing. Allergy Asthma Immunol Res. 2019;11(2):231-240. [5]. Eperisone Hydrochloride, a Muscle Relaxant, Is a Potent P2X7 Receptor Antagonist. Chem Pharm Bull (Tokyo). 2024;72(3):345-348. |
| 其他信息 |
Eperisone hydrochloride is an aromatic ketone.
Although the exact pathogenesis of idiopathic pulmonary fibrosis (IPF) is still unknown, the transdifferentiation of fibroblasts into myofibroblasts and the production of extracellular matrix components such as collagen, triggered by alveolar epithelial cell injury, are important mechanisms of IPF development. In the lungs of IPF patients, apoptosis is less likely to be induced in fibroblasts than in alveolar epithelial cells, and this process is involved in the pathogenesis of IPF. We used a library containing approved drugs to screen for drugs that preferentially reduce cell viability in LL29 cells (lung fibroblasts from an IPF patient) compared with A549 cells (human alveolar epithelial cell line). After screening, we selected eperisone, a central muscle relaxant used in clinical practice. Eperisone showed little toxicity in A549 cells and preferentially reduced the percentage of viable LL29 cells, while pirfenidone and nintedanib did not have this effect. Eperisone also significantly inhibited transforming growth factor-β1-dependent transdifferentiation of LL29 cells into myofibroblasts. In an in vivo study using ICR mice, eperisone inhibited bleomycin (BLM)-induced pulmonary fibrosis, respiratory dysfunction, and fibroblast activation. In contrast, pirfenidone and nintedanib were less effective than eperisone in inhibiting BLM-induced pulmonary fibrosis under this experimental condition. Finally, we showed that eperisone did not induce adverse effects in the liver and gastrointestinal tract in the BLM-induced pulmonary fibrosis model. Considering these results, we propose that eperisone may be safer and more therapeutically beneficial for IPF patients than current therapies. [1] Although pirfenidone and nintedanib are currently used in clinical practice to treat IPF, in some cases, these drugs have not shown efficacy and have been reported to induce adverse effects such as elevation of liver damage markers, diarrhea, and indigestion. Thus, in this study, we conducted a “drug-repositioning strategy” to identify safer and more effective drugs for IPF treatment. The in vitro studies shown in Figs. 1 and 2 revealed that eperisone, but not pirfenidone or nintedanib, exhibited a fibroblast-preferential reduction of viable cells. Moreover, the in vivo studies shown in Fig. 3 and Supplementary Fig. S1 indicated that eperisone, but not pirfenidone or nintedanib, inhibited the exacerbation of BLM-induced pulmonary fibrosis. In addition, eperisone did not induce adverse effects such as hepatotoxicity marker elevation or gastrointestinal disorders. Therefore, we suggest that eperisone may be a safer and more effective treatment for IPF than pirfenidone or nintedanib. After screening drugs that selectively induce fibroblast cell death, we selected eperisone and showed its efficacy in animal models of IPF, which is caused by fibroblast activation. As mentioned above, eperisone has never been reported to preferentially induce cell death in fibroblasts or effectively treat fibrosis models. However, fibrosis is also induced in organs other than the lungs, such as the liver, heart, and kidneys. For example, in the liver, hepatic stellate cells are activated by stimuli such as TGF-β1 and transdifferentiate into myofibroblasts, which promote the production of extracellular matrix components such as collagen and induce liver fibrosis in diseases such as nonalcoholic steatohepatitis. In the kidney, resident fibroblasts, pericytes, bone marrow-derived cells, and endothelial cells transdifferentiate into myofibroblasts and induce kidney fibrosis. Thus, activated myofibroblasts that transdifferentiate from fibroblasts play a role in promoting fibrosis in organs other than the lungs. Therefore, eperisone, which can preferentially inhibit fibroblast activity, may be effective not only in lung fibrosis models but also in fibrosis models of other organs; thus, the results of this study have promising applications for future research.[1] |
| 分子式 |
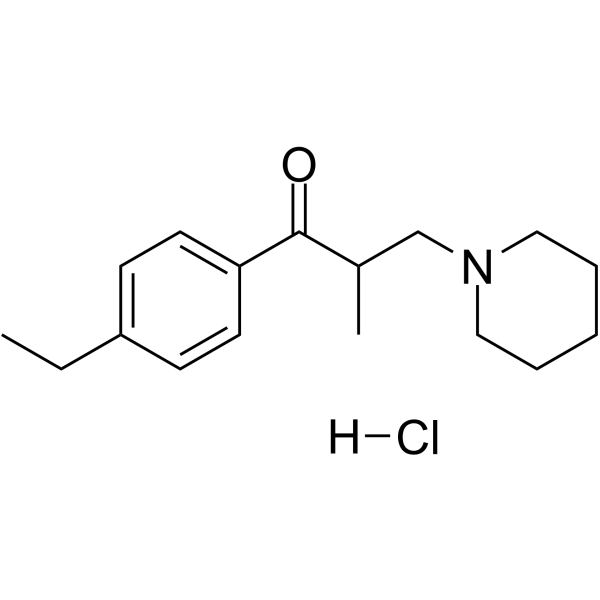
C₁₇H₂₆CLNO
|
|---|---|
| 分子量 |
295.85
|
| 精确质量 |
295.17
|
| 元素分析 |
C, 69.02; H, 8.86; Cl, 11.98; N, 4.73; O, 5.41
|
| CAS号 |
56839-43-1
|
| 相关CAS号 |
Eperisone-d10 hydrochloride;1246819-46-4;Eperisone;64840-90-0
|
| PubChem CID |
123698
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
0.994g/cm3
|
| 沸点 |
386.8ºC at 760mmHg
|
| 熔点 |
168-174ºC
|
| 闪点 |
137.4ºC
|
| LogP |
4.293
|
| tPSA |
20.31
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
20
|
| 分子复杂度/Complexity |
275
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CCC1=CC=C(C=C1)C(=O)C(C)CN2CCCCC2.Cl
|
| InChi Key |
GTAXGNCCEYZRII-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C17H25NO.ClH/c1-3-15-7-9-16(10-8-15)17(19)14(2)13-18-11-5-4-6-12-18;/h7-10,14H,3-6,11-13H2,1-2H3;1H
|
| 化学名 |
1-(4-ethylphenyl)-2-methyl-3-piperidin-1-ylpropan-1-one;hydrochloride
|
| 别名 |
Eperisone hydrochloride; 56839-43-1; eperisone; 64840-90-0; Eperisone [INN]; Eperisona; (+-)-Eperisone; Eperisonum; Eperisonum [INN-Latin]; Eperisona [INN-Spanish]; Eperisone HCl; Myonal; Mional; E-646; UNII-U38O8U7P6X; Epenard (TN);
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ~100 mg/mL (~338.01 mM)
DMSO : ~31.25 mg/mL (~105.63 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (7.03 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (7.03 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (7.03 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 16.67 mg/mL (56.35 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.3801 mL | 16.9005 mL | 33.8009 mL | |
| 5 mM | 0.6760 mL | 3.3801 mL | 6.7602 mL | |
| 10 mM | 0.3380 mL | 1.6900 mL | 3.3801 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。