| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Human Mineralocorticoid Receptor (MR) (Ki = 0.1 nM, determined by radioligand binding assay) [1]
- Glucocorticoid Receptor (GR) (Ki = 260 nM, determined by radioligand binding assay; >2600-fold selectivity for MR) [1] - Progesterone Receptor (PR)/Androgen Receptor (AR) (Ki > 1000 nM, no significant binding) [1] |
|---|---|
| 体外研究 (In Vitro) |
eplerenone 抑制人盐皮质激素受体,IC50 值为 0.081 μM[2]。
强效选择性MR拮抗剂:依普利酮(Eplerenone; Epoxymexrenone; CGP 30083)竞争性抑制[3H]-醛固酮与人MR的结合,Ki = 0.1 nM,对GR的选择性>2600倍,对PR/AR无显著结合[1] - 降低巨噬细胞氧化应激:1 μM 依普利酮使人外周血单核细胞中LPS诱导的活性氧(ROS)生成减少约60%,NADPH氧化酶活性降低约55%[3] - 抑制促炎细胞因子释放:10 μM 依普利酮使LPS刺激的小鼠巨噬细胞中TNF-α和IL-6分泌分别减少约45%和40%[3] - 浓度高达100 μM时,对血管平滑肌细胞(VSMCs)或单核细胞无细胞毒性(细胞存活率>90%)[3] |
| 体内研究 (In Vivo) |
在动脉粥样硬化载脂蛋白缺陷(EO)小鼠中,口服依普利酮(200 mg/kg/天)三个月可显着降低氧化应激和动脉粥样硬化的进展[3]。
轻中度高血压患者的降压活性:口服依普利酮(50-200 mg/天,持续8周),剂量依赖性降低收缩压(SBP)8-15 mmHg、舒张压(DBP)5-9 mmHg,200 mg/天达到最大疗效[1] - 慢性收缩性心力衰竭患者的疗效:口服25-50 mg/天 依普利酮,较安慰剂降低心血管死亡率约30%,心力衰竭住院率约20%[2] - 减轻ApoE缺陷小鼠的动脉粥样硬化:口服依普利酮(100 mg/kg/天,持续16周),主动脉粥样硬化斑块面积减少约45%,血清氧化应激标志物(MDA、8-异前列腺素F2α)降低50-60%,斑块内巨噬细胞浸润减少约55%[3] - 降低高血压大鼠的血管氧化应激:口服10 mg/kg/天 依普利酮,主动脉NADPH氧化酶活性降低约40%,ROS水平降低约45%[3] |
| 酶活实验 |
MR放射配体结合实验:重组人MR蛋白固定于微量滴定板,与[3H]-醛固酮(0.5 nM)及系列稀释的依普利酮(0.001-1000 nM)在结合缓冲液中孵育。4°C孵育18小时后,反复洗涤去除未结合配体,液体闪烁计数法测量结合部分的放射性强度,通过竞争结合分析计算Ki值[1]
- GR/PR/AR选择性实验:重组人GR、PR、AR蛋白采用与MR相同的放射配体结合方案,分别使用对应的[3H]标记配体(GR用地塞米松,PR用孕酮,AR用二氢睾酮)。量化结合抑制率以评估亚型选择性[1] |
| 细胞实验 |
巨噬细胞氧化应激实验:分离人外周血单核细胞并分化为巨噬细胞,用依普利酮(0.1-10 μM)预处理2小时,再用LPS(1 μg/mL)刺激24小时。荧光探针检测ROS生成,NADPH消耗实验评估NADPH氧化酶活性[3]
- 细胞因子分泌实验:小鼠骨髓来源巨噬细胞接种于24孔板,用依普利酮(1-100 μM)预处理1小时,LPS(1 μg/mL)刺激24小时。收集培养上清液,ELISA法定量TNF-α/IL-6水平[3] - 血管平滑肌细胞活力实验:血管平滑肌细胞接种于96孔板,用依普利酮(0.1-100 μM)处理72小时,MTT法检测细胞活力以评估细胞毒性[3] |
| 动物实验 |
Animal/Disease Models: Atherosclerotic apolipoprotein Edeficient (EO) mice[3]
Doses: 200 mg/kg Route of Administration: po (oral gavage) 200 mg/kg/day for 3 months Experimental Results: Dramatically diminished systolic and diastolic blood pressure by 12% and 11%, respectively. diminished serum susceptibility to lipid peroxidation by as much as 26%, and increased serum paraoxonase activity by 28%. decreased levels of lipid peroxides, and Dramatically decreased macrophage oxidation of low-density lipoprotein (LDL) and superoxide ion release. Dramatically decreased the atherosclerotic lesion area. Mild-to-moderate hypertension human clinical trial: Patients with SBP 140-179 mmHg and DBP 90-109 mmHg were randomized to placebo or Eplerenone (50, 100, 200 mg/day) oral administration for 8 weeks. Blood pressure was measured at baseline and weekly using standardized sphygmomanometry; serum electrolytes and renal function were monitored [1] - ApoE-deficient mouse atherosclerosis model: 6-week-old ApoE-/- mice were fed a high-fat diet and randomized to vehicle or Eplerenone (50, 100 mg/kg/day) oral gavage for 16 weeks. Aortic atherosclerotic plaque area was quantified by Oil Red O staining; serum MDA, 8-iso-PGF2α, and pro-inflammatory cytokines were measured [3] - Chronic systolic heart failure clinical trial: Patients with left ventricular ejection fraction ≤35% and mild symptoms (NYHA class II) were treated with Eplerenone (25-50 mg/day, oral) for 12 months. Primary endpoints included cardiovascular mortality and heart failure hospitalization; secondary endpoints included left ventricular remodeling and functional capacity [2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The absolute bioavailability of eplerenone is unknown. 43 to 90 L Apparent plasma cl=10 L/hr Apparent plasma clearance: approximately 10 L/hr. Less than 5% is recovered as unchanged drug in the urine and feces. Renal: 67%. Fecal: 32%. Mean peak plasma concentrations of eplerenone are reached approximately 1.5 hours following oral administration. The absolute bioavailability of eplerenone is unknown. Both peak plasma levels (Cmax) and area under the curve (AUC) are dose proportional for doses of 25 to 100 mg and less than proportional at doses above 100 mg. The plasma protein binding of eplerenone is about 50% and it is primarily bound to alpha 1-acid glycoproteins. The apparent volume of distribution at steady state ranged from 43 to 90 L. Eplerenone does not preferentially bind to red blood cells. Eplerenone is distributed into milk in rats; ... . ... Preclinical data show that eplerenone and/or metabolites are present in rat breast milk (0.85:1 [milk:plasma] AUC ratio) obtained after a single oral dose. Peak concentrations in plasma and milk were obtained from 0.5 to 1 hour after dosing. Metabolism / Metabolites Eplerenone is metabolized primarily by CYP3A4, however, no active metabolites have been identified in human plasma. Eplerenone metabolism is primarily mediated via CYP3A4. No active metabolites have been identified in human plasma. Eplerenone has known human metabolites that include 21-hydroxyeplerenone and 6beta-hydroxyeplerenone. Biological Half-Life 4-6 hours Elimination: 4 to 6 hours. Oral bioavailability: ~50% (human); absorption is not affected by food [2] - Plasma half-life (t1/2): 4-6 hours (human) [2] - Peak plasma concentration (Cmax): 133 ng/mL (human, 100 mg oral) [2] - Volume of distribution (Vd): 43 L (human) [2] - Metabolism: Primarily metabolized by cytochrome P450 3A4; major metabolites are inactive [2] - Excretion: ~67% excreted in feces (as metabolites), ~32% in urine (as metabolites); unchanged drug < 5% [2] - Plasma protein binding rate: ~50% (human) [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Eplerenone therapy has been associated with a low rate of serum aminotransferase elevations which are typically mild and transient. ALT elevations of greater than 3 times the ULN occurred in 0.7% and greater than 5 times in 0.2% of eplerenone treated compared to 0.3% and 0.3% of placebo treated subjects. Idiosyncratic, clinically apparent liver injury from eplerenone has yet to be reported. The similarity in structure to spironolactone suggests that it may share susceptibility to the acute liver injury reported rarely with that agent. Likelihood score: E (unproven but suspect rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Data from one mother-infant pair indicate that eplerenone is poorly excreted into breastmilk. Until more data are available, eplerenone should be used with careful infant monitoring during breastfeeding. ◉ Effects in Breastfed Infants A woman with primary aldosteronism was receiving eplerenone 50 mg once daily (0.79 mg/kg daily) during pregnancy and postpartum. Her infant was partially breastfed for 3 months, with over 50% of nutrition from breastmilk. The infant developed normally and had no detectable adverse drug effects at the 1- or 3-month checkups. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding 50% Interactions Lithium toxicity has been reported in patients receiving lithium concomitantly with diuretics and ACE inhibitors; serum lithium concentrations should be monitored if eplerenone is administered concomitantly with lithium. Antihypertensive and/or diuretic effects may be potentiated when these medications /other hypotension-producing medications/ are used concurrently with eplerenone; although some antihypertensive and/or diuretic combinations are frequently used for therapeutic advantage, dosage adjustments may be necessary during concurrent use. /Use of grapefruit juice with eplerenone/ may cause a small increase in exposure. Concomitant use of potent inhibitors of CYP450 3A4 /including eltraconazole or ketoconazole/ with eplerenone is contraindicated. For more Interactions (Complete) data for EPLERENONE (12 total), please visit the HSDB record page. Electrolyte disturbance: Hyperkalemia (serum potassium >5.5 mmol/L) reported in ~3.5% of patients; risk increased in renal impairment or concurrent use of ACE inhibitors [2] - Renal toxicity: No significant changes in serum creatinine or estimated glomerular filtration rate (eGFR) in patients with normal renal function [1, 2] - Hepatic toxicity: No elevation in ALT/AST levels in clinical trials; safe in patients with mild-to-moderate hepatic impairment [2] - Acute toxicity: LD50 > 2000 mg/kg (oral in rat and mouse) [1] - Clinical adverse events: Headache (7%), dizziness (5%), fatigue (4%); adverse event rate similar to placebo [1, 2] |
| 参考文献 |
[1]. Myron H Weinberger, et al. Eplerenone, a selective aldosterone blocker, in mild-to-moderate hypertension. Am J Hypertens. 2002 Aug;15(8):709-16.
[2]. Dhillon, S., Eplerenone: a review of its use in patients with chronic systolic heart failure and mild symptoms. Drugs, 2013. 73(13): p. 1451-62. [3]. Shlomo Keidar, et al. Effect of eplerenone, a selective aldosterone blocker, on blood pressure, serum and macrophage oxidative stress, and atherosclerosis in apolipoprotein E-deficient mice. J Cardiovasc Pharmacol. 2003 Jun;41(6):955-63. |
| 其他信息 |
Eplerenone is a steroid acid ester, a methyl ester, an oxaspiro compound, a gamma-lactone, an organic heteropentacyclic compound, a 3-oxo-Delta(4) steroid and an epoxy steroid. It has a role as an aldosterone antagonist and an antihypertensive agent. It derives from a hydride of a pregnane.
Eplerenone, an aldosterone receptor antagonist similar to spironolactone, has been shown to produce sustained increases in plasma renin and serum aldosterone, consistent with inhibition of the negative regulatory feedback of aldosterone on renin secretion. The resulting increased plasma renin activity and aldosterone circulating levels do not overcome the effects of eplerenone. Eplerenone selectively binds to recombinant human mineralocorticoid receptors relative to its binding to recombinant human glucocorticoid, progesterone and androgen receptors. Eplerenone is an Aldosterone Antagonist. The mechanism of action of eplerenone is as an Aldosterone Antagonist. Eplerenone is an aldosterone receptor antagonist and potassium-sparing diuretic used in the therapy of hypertension. Eplerenone therapy has been associated with transient elevations in serum aminotransferase levels, but has yet to be linked to cases of clinically apparent drug induced liver disease. Eplerenone is a selective aldosterone receptor antagonist. Eplerenone binds to the mineralocorticoid receptor and blocks the binding of aldosterone, thereby decreasing sodium resorption and subsequently increasing water outflow. This leads to a decrease in blood pressure. Eplerenone is used in the treatment of hypertension and congestive heart failure. A spironolactone derivative and selective ALDOSTERONE RECEPTOR antagonist that is used in the management of HYPERTENSION and CONGESTIVE HEART FAILURE, post-MYOCARDIAL INFARCTION. Drug Indication For improvement of survival of stable patients with left ventricular systolic dysfunction (ejection fraction <40%) and clinical evidence of congestive heart failure after an acute myocardial infarction. FDA Label Mechanism of Action Eplerenone binds to the mineralocorticoid receptor and thereby blocks the binding of aldosterone (component of the renin-angiotensin-aldosterone-system, or RAAS). Aldosterone synthesis, which occurs primarily in the adrenal gland, is modulated by multiple factors, including angiotensin II and non-RAAS mediators such as adrenocorticotropic hormone (ACTH) and potassium. Aldosterone binds to mineralocorticoid receptors in both epithelial (e.g., kidney) and nonepithelial (e.g., heart, blood vessels, and brain) tissues and increases blood pressure through induction of sodium reabsorption and possibly other mechanisms. Eplerenone has relative selectivity in binding to recombinant human mineralocorticoid receptors compared to its binding to recombinant human glucocorticoid, progesterone, and androgen receptors. Eplerenone has been shown to produce sustained increases in plasma renin and serum aldosterone, consistent with the inhibition of the negative regulatory feedback of aldosterone on renin secretion. The resulting increased plasma renin activity and aldosterone circulation levels do not overcome the effect of eplerenone on blood pressure. Eplerenone binds to the mineralocorticoid receptor and blocks the binding of aldosterone, a component of the renin-angiotensin-aldosterone-system (RAAS). Aldosterone synthesis, which occurs primarily in the adrenal gland, is modulated by multiple factors, including angiotensin II and non-RAAS mediators such as adrenocorticotropic hormone (ACTH) and potassium. Aldosterone binds to mineralocorticoid receptors in both epithelial (e.g., kidney) and nonepithelial (e.g., heart, blood vessels, brain) tissues and increases blood pressure through induction of sodium resorption and possibly other mechanisms. Therapeutic Uses Eplerenone is indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensive drugs. /Included in US product labeling/ Inspra is indicated to improve survival of stable patients with left ventricular systolic dysfunction (ejection fraction less than or equal to 40%) and clinical evidence of congestive heart failure after an acute myocardial infarction. ... Eplerenone should replace spironolactone as a natriuretic and antikaliuretic in heart failure and as add-on treatment in severe systolic cardiac insufficiency, and it is indicated after an acute myocardial infarction complicated by left ventricular dysfunction and heart failure. The finding that hypertension control with diuretic-based pharmacotherapy results in better prevention of heart failure than pressure reduction with other drugs makes it pertinent to investigate whether diuretics in general, and eplerenone in particular, should constitute part of the initial pharmacotherapy for heart failure when there is no overt fluid retention and independent of the etiology. ... Drug Warnings FDA Pregnancy Risk Category: B /NO EVIDENCE OF RISK IN HUMANS. Adequate, well controlled studies in pregnant women have not shown increased risk of fetal abnormalities despite adverse findings in animals, or, in the absence of adequate human studies, animal studies show no fetal risk. The chance of fetal harm is remote but remains a possibility./ ... When used for hypertension, the drug is contraindicated in patients with type 2 diabetes mellitus with microalbuminuria, serum creatinine concentrations exceeding 2 or 1.8 mg/dL in males or females, respectively, creatinine clearance less than 50 mL/minute, ... . The most serious risk associated with eplerenone therapy is hyperkalemia (serum potassium greater than 5.5 mEq/L), which may cause serious, sometimes fatal, cardiac arrhythmias. Patients with impaired renal function or diabetes mellitus and patients receiving concurrent agents affecting the renin-angiotensin-aldosterone system (e.g., angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor antagonists) are at an increased risk for developing hyperkalemia. Eplerenone should be used with caution in patients with congestive heart failure following an acute myocardial infarction, who have renal impairment (i.e., serum creatinine concentrations exceeding 2 or 1.8 mg/dL in males or females, respectively, or creatinine clearance of 50 mL/minute or less) or those with diabetes mellitus (including those with proteinuria). Serum potassium concentrations should be monitored periodically in patients receiving eplerenone. Dosage reduction has been shown to decrease serum potassium concentrations. Adverse effects reported in 1% or more of patients receiving eplerenone for the management of hypertension are dizziness, fatigue, flu-like symptoms, cough, diarrhea, abdominal pain, hyperkalemia, decreased serum sodium concentrations, abnormal vaginal bleeding, gynecomastia, hypercholesterolemia, hypertriglyceridemia, mastodynia, or albuminuria. For more Drug Warnings (Complete) data for EPLERENONE (12 total), please visit the HSDB record page. Pharmacodynamics Eplerenone, an aldosterone receptor antagonist similar to spironolactone, has been shown to produce sustained increases in plasma renin and serum aldosterone, consistent with inhibition of the negative regulatory feedback of aldosterone on renin secretion. The resulting increased plasma renin activity and aldosterone circulating levels do not overcome the effects of eplerenone. Eplerenone selectively binds to recombinant human mineralocorticoid receptors relative to its binding to recombinant human glucocorticoid, progesterone and androgen receptors. Eplerenone (Epoxymexrenone; CGP 30083) is a selective oral mineralocorticoid receptor (MR) antagonist [1, 2, 3] - Core mechanism of action: Competes with aldosterone for binding to MR in renal tubules (promoting sodium excretion and potassium retention) and vascular tissues (reducing oxidative stress, inflammation, and fibrosis) [1, 3] - Approved indications: Mild-to-moderate hypertension; chronic systolic heart failure (NYHA class II) to reduce cardiovascular mortality and hospitalization [1, 2] - Selectivity advantage: Higher selectivity for MR over GR (vs. spironolactone) reduces anti-androgenic side effects (e.g., gynecomastia, impotence) [1] - Clinical use: Recommended starting dose of 25 mg/day for heart failure, titrated to 50 mg/day; 50-100 mg/day for hypertension [2] |
| 分子式 |
C24H30O6
|
|
|---|---|---|
| 分子量 |
414.49
|
|
| 精确质量 |
414.204
|
|
| CAS号 |
107724-20-9
|
|
| 相关CAS号 |
Eplerenone-d3
|
|
| PubChem CID |
443872
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
597.9±50.0 °C at 760 mmHg
|
|
| 熔点 |
241-243ºC
|
|
| 闪点 |
259.5±30.2 °C
|
|
| 蒸汽压 |
0.0±1.7 mmHg at 25°C
|
|
| 折射率 |
1.587
|
|
| LogP |
1.05
|
|
| tPSA |
82.2
|
|
| 氢键供体(HBD)数目 |
0
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
2
|
|
| 重原子数目 |
30
|
|
| 分子复杂度/Complexity |
907
|
|
| 定义原子立体中心数目 |
8
|
|
| SMILES |
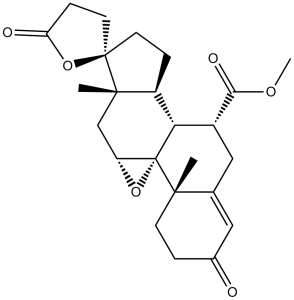
C[C@]12CCC(=O)C=C1C[C@H]([C@@H]3[C@]24[C@H](O4)C[C@]5([C@H]3CC[C@@]56CCC(=O)O6)C)C(=O)OC
|
|
| InChi Key |
JUKPWJGBANNWMW-VWBFHTRKSA-N
|
|
| InChi Code |
InChI=1S/C24H30O6/c1-21-7-4-14(25)10-13(21)11-15(20(27)28-3)19-16-5-8-23(9-6-18(26)30-23)22(16,2)12-17-24(19,21)29-17/h10,15-17,19H,4-9,11-12H2,1-3H3/t15-,16+,17-,19+,21+,22+,23-,24-/m1/s1
|
|
| 化学名 |
methyl (1R,2S,9R,10R,11S,14R,15S,17R)-2,15-dimethyl-5,5'-dioxospiro[18-oxapentacyclo[8.8.0.01,17.02,7.011,15]octadec-6-ene-14,2'-oxolane]-9-carboxylate
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.03 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.03 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.03 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4126 mL | 12.0630 mL | 24.1260 mL | |
| 5 mM | 0.4825 mL | 2.4126 mL | 4.8252 mL | |
| 10 mM | 0.2413 mL | 1.2063 mL | 2.4126 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
The Predictive Role of Urinary Proteomics in Blood Pressure Response of Obese Hypertensive Treated With Irbesartan or Eplerenone.
CTID: NCT06208072
Phase: N/A Status: Recruiting
Date: 2024-01-17