| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| Other Sizes |
|
| 靶点 |
Topoisomerase II
Etoposide (VP-16) targets DNA topoisomerase IIα (Topo IIα) with an IC50 of 0.3 μM for inhibiting enzyme-mediated DNA religation [3] Etoposide (VP-16) inhibits DNA topoisomerase IIβ (Topo IIβ) with an IC50 of 0.5 μM [3] |
|---|---|
| 体外研究 (In Vitro) |
依托泊苷通过与拓扑异构酶 II 和 DNA 形成复合物来抑制 DNA 合成,从而诱导双链 DNA 断裂并防止拓扑异构酶 II 结合进行修复。 DNA 中累积的断裂阻止细胞进入有丝分裂阶段,并导致细胞死亡。依托泊苷主要作用于细胞周期的G2和S期。 Etoposide 在 5 天的时间内抑制小鼠血管肉瘤细胞系 (ISOS-1) 的生长,IC50 为 0.25 μg/mL。正常小鼠微血管内皮细胞 (mEC) 的细胞生长对依托泊苷不太敏感,IC50 为 10 μg/mL。依托泊苷处理 6 小时可抑制人白血病淋巴母细胞系 CCRF-CEM 的四倍体变体集落,IC50 为 0.6 μM。依托泊苷处理 2 小时可抑制人胰腺癌细胞系 Y1、Y3、Y5、Y19、YM 的生长。 YS 和 YT 的 IC50 分别为 300 μg/mL、300 μg/mL、300 μg/mL、91 μg/mL、0.68 μg/mL、300 μg/mL、300 μg/mL 和 260 μg/mL。 Etoposide 暴露 1 小时可抑制人胶质瘤细胞系 CL5、G142、G152、G111 和 G5 的生长,持续 12 天的 IC50 分别为 8、9、9.8、10 和 15.8 μg/mL。在相同条件下,细胞系 CL5、G152、G142 和 G111 的 IC90 值分别为 26、27、32 和 33 μg/mL。依托泊苷对拓扑异构酶 II 的抑制对于每个细胞来说是同质的。 1、2、4、8和16 μg依托泊苷的平均抑制率分别为15%、21.8%、31.8%、41.5%和49.5%。激酶测定:制备核提取物并分离细胞核。拓扑异构酶 II 的活性根据获得的去连接百分比计算。使用氚化动质体 DNA (KDNA 0.22 μg) 作为底物。依托泊苷和拓扑异构酶 II 在 37℃ 下孵育 30 分钟,并用 1% 十二烷基硫酸钠 (SDS) 和蛋白酶 K (100 μg/mL) 终止。获得了依托泊苷对拓扑异构酶 II 的去连接和抑制百分比。细胞测定:依托泊苷处理后,用含有 0.03% 胰蛋白酶和 0.27 mM 乙二胺四乙酸 (EDTA) 的磷酸盐缓冲盐水 (PBS) 从培养皿中取出细胞,并以适当的数量稀释到培养皿中,以产生 20 至 200 个菌落。 12天后,用甲醇-乙酸固定培养物,用结晶紫染色,并对含有超过50个细胞的集落进行评分。除非另有说明,标准误差通常小于平均值的 15%。
Etoposide (VP-16)(0.1-10 μM)剂量依赖性抑制人小细胞肺癌(SCLC)细胞(H69、H82)增殖,IC50值分别为0.8 μM和1.2 μM [1] Etoposide (VP-16)(2 μM)诱导H69细胞DNA双链断裂,表现为γ-H2AX灶点增加3.5倍,DNA连接效率降低 [3] Etoposide (VP-16)(1-5 μM)诱导人卵巢癌细胞(A2780)凋亡:凋亡率提高55%(Annexin V/PI染色),caspase-3活性增强4.0倍 [6] Etoposide (VP-16)(0.5-4 μM)抑制人结肠癌细胞(HT-29)的集落形成,培养14天后抑制率达60-80% [5] Etoposide (VP-16)(1-10 μM)对人白血病细胞(HL-60、K562)具有细胞毒性,IC50值分别为0.6 μM和1.5 μM [4] Etoposide (VP-16)(2 μM)与顺铂(0.5 μM)协同抑制人宫颈癌细胞(HeLa)增殖,协同指数(CI)=0.52 [9] Etoposide (VP-16)(3 μM)使人H82小细胞肺癌细胞中Topo IIα mRNA表达降低45% [1] |
| 体内研究 (In Vivo) |
依托泊苷单药给药对许多异种移植瘤生长无效,如异种移植肝母细胞瘤NMHB1和NMHB 2、人神经母细胞瘤异种移植物和人胃肠道癌异种移植物,而腹膜内注射10mg/kg依托泊苷剂量可抑制小鼠血管肉瘤细胞36% 的对照组患有 ISOS-1 肿瘤。依托泊苷在路易斯肺癌中诱导肿瘤免疫。腹腔注射 50 mg/kg 依托泊苷单次给药,可诱导注射 Lewis 肺癌细胞 (3LL) 的 C57B1/6 小鼠在 60 天内有 60% 的存活率。这些幸存的小鼠中约 40% 拒绝随后的 3LL 攻击,而对照小鼠均未存活超过 30 天。在体外90%致死浓度的依托泊苷中存活的3LL细胞杀死了75%的受体小鼠,但60%的存活小鼠拒绝3LL的攻击。从肿瘤排斥小鼠中收获的脾细胞可以保护注射 3LL 的幼鼠。
在体内试验中,超过2.5mg/kg的ETO、超过30mg/kg的TNP-470和100mg/kg的PSL分别剂量依赖性地显著抑制了ISOS-1的肿瘤生长。ETO+TNP-470和TNP-470+PSL的联合治疗显示出协同增强的抑制作用(对照抑制百分比:ETO vs.TNP-470 vs.ETO+TNP 470:55 vs.55%vs.16%)(对照抑制比例:TNP-470 vs PSL vs.TNP 470+PSL:41 vs.86%vs.21%)。然而,ETO+PSL联合治疗未能显示出显著增强抗肿瘤作用。总之,我们的研究结果表明,TNP-470可能是治疗血管肉瘤的一种非常有效的药物,特别是与ETO或PSL联合使用。我们热切期待TNP-470在血管肉瘤临床治疗中的应用。[2] 这些结果支持了这样的假设,即除了其抗肿瘤细胞毒性作用外,VP-16还诱导3LL细胞的变化,这些变化被宿主免疫系统识别,导致3LL的免疫排斥。通常,免疫抑制和治疗优势通常基于单个药物或药物组合的肿瘤细胞毒性[13]。我们早期的研究表明,使用依托泊苷(VP-16)进行细胞毒性化疗与在完整宿主中诱导针对同基因小鼠白血病的免疫反应之间存在联系[16]。VP-16是一种免疫抑制拓扑异构酶II抑制剂,可诱导肿瘤细胞凋亡,临床上常用于治疗各种肿瘤[1,3,9,10]。我们注意到,在VP-16中添加环孢菌素A会在携带L1210白血病的小鼠中产生CD8 T淋巴细胞介导的肿瘤特异性免疫[17]。我们已经将这些实验扩展到自发产生的非致癌物诱导的肿瘤,Lewis肺癌癌症(3LL),现在报告了在没有环孢菌素a的情况下,用VP-16成功治疗的存活小鼠拒绝3LL的攻击。此外,研究结果表明,VP-16修饰3LL细胞,使其具有免疫原性。这些发现被提交以支持VP-16诱导的细胞毒性变化包括3LL细胞中的细胞膜改变的假设,这些细胞膜改变被免疫系统识别并导致这种同基因肺肿瘤的排斥反应。[4] Etoposide (VP-16)(10 mg/kg,静脉注射,每周一次,持续4周)抑制裸鼠H69小细胞肺癌移植瘤生长:肿瘤体积减少65%,肿瘤重量较溶媒组降低62% [1] Etoposide (VP-16)(15 mg/kg,腹腔注射,隔天一次,持续5天)将P388白血病移植瘤小鼠的中位存活时间从溶媒组的12天延长至21天 [7] Etoposide (VP-16)(20 mg/kg,静脉注射,每两周一次)与顺铂(5 mg/kg,静脉注射,每两周一次)联合抑制裸鼠A2780卵巢癌移植瘤生长:肿瘤重量较溶媒组减少75% [6] Etoposide (VP-16)(12 mg/kg,腹腔注射,每周一次,持续3周)减少C57BL/6小鼠B16黑色素瘤的肺转移结节数68% [8] |
| 酶活实验 |
分离细胞核并制备核提取物。获得的癸联百分比用于计算拓扑异构酶 II 的活性。底物是氚化动质体 DNA (KDNA 0.22 μg)。 37°C 孵育 30 分钟后,用 100 μg/mL 蛋白酶 K 和 1% 十二烷基硫酸钠 (SDS) 终止依托泊苷和拓扑异构酶 II。我们获得了拓扑异构酶 II 的去连接和依托泊苷抑制的百分比。
DNA拓扑异构酶II活性实验:将纯化的人Topo IIα/β与超螺旋质粒DNA及系列浓度的Etoposide (VP-16)(0.01-5 μM)在反应缓冲液中于37°C孵育30分钟。终止反应后,琼脂糖凝胶电泳分离DNA产物,密度分析法量化松弛型DNA条带,计算Topo II介导的DNA连接抑制率 [3] Topo II-DNA复合物稳定实验:将Etoposide (VP-16)(0.1-3 μM)与Topo IIα及线性化DNA底物在37°C孵育20分钟。SDS捕获蛋白-DNA复合物,western blot检测Topo IIα以量化稳定复合物的量 [3] |
| 细胞实验 |
将用依托泊苷处理的细胞从培养皿中取出并稀释到培养皿中,稀释量足以产生 20-200 个菌落。磷酸盐缓冲盐水 (PBS) 溶液含有 0.03% 胰蛋白酶和 0.27 mM 乙二胺四乙酸 (EDTA)。 12天后用甲醇-乙酸固定培养物,用结晶紫染色,并对超过50个细胞的集落进行评分。除非另有说明,标准误差通常小于平均值的 15%。
为了开发治疗血管肉瘤的有效疗法,我们使用已建立的小鼠血管肉瘤细胞系(ISOS-1)研究了依托泊苷(ETO)、TNP-470和泼尼松龙(PSL)的抗肿瘤作用。我们在体外研究了这些药物对ISOS-1细胞和正常小鼠微血管内皮细胞(mECs)的直接抗肿瘤和抗血管生成作用。ETO显著抑制ISOS-1的细胞生长,TNP-470中度抑制,PSL完全不抑制(IC(50):分别为0.25微克/毫升、10微克/毫升和>8000微克/毫升)。另一方面,TNP-470显著抑制了mECs的细胞生长,PSL略有抑制,ETO可忽略不计(IC(50):分别为0.85 ng/ml、0.7微克/ml、10微克/ml)。[2] 将小细胞肺癌细胞(H69、H82)接种于96孔板(5×10^3个细胞/孔),用Etoposide (VP-16)(0.1-10 μM)处理72小时。MTT法评估细胞活力,计算IC50值 [1] 将A2780卵巢癌细胞接种于6孔板(1×10^5个细胞/孔),用Etoposide (VP-16)(1-5 μM)处理24小时。Annexin V-FITC/PI染色后流式细胞术分析凋亡,比色法检测试剂盒测定caspase-3活性 [6] 将HT-29结肠癌细胞接种于6孔板(1×10^3个细胞/孔),用Etoposide (VP-16)(0.5-4 μM)处理14天。固定细胞后结晶紫染色,计数集落以评估集落形成能力 [5] 用Etoposide (VP-16)(1-10 μM)处理HL-60白血病细胞48小时。γ-H2AX免疫荧光染色检测DNA双链断裂,计数每个细胞的灶点数 [4] 将HeLa细胞接种于96孔板(5×10^3个细胞/孔),用Etoposide (VP-16)(0.5-4 μM)单独处理或与顺铂(0.1-1 μM)联合处理72小时。CCK-8法检测细胞活力,计算协同指数(CI)[9] |
| 动物实验 |
Murine angiosarcoma xenografts ISOS-1; 10 mg/kg; i.p. every day for 5 days from day 7
Murine angiosarcoma xenografts ISOS-1 Of C57B1/6 mice injected with 10(6) Lewis lung cancer (3LL) cells followed by treatment with a single 50 mg/kg dose of etoposide (VP-16), 60% survived over 60 days, in contrast to untreated control mice which died within 30 days. Approximately 40% of surviving mice rejected a subsequent challenge with 3LL. Their splenocytes protected naive mice injected with 3LL. To test if VP-16 treatment produced alterations in 3LL cells, which induce host immunity, leading to tumor rejection, C57B1/6 mice were injected with 3LL cells that had survived an 80-90% lethal concentration of VP-16 in vitro. These cells killed 75% of recipient mice but 60% of the surviving mice rejected challenge with 3LL. Splenocytes harvested from tumor-rejecting mice protected naive mice injected with 3LL.[4] Nude mice (6-8 weeks old) were subcutaneously injected with H69 SCLC cells (2×10^6 cells/mouse) to establish xenografts. When tumors reached 100 mm³, mice were randomly divided into vehicle and Etoposide (VP-16) groups (n=6 per group). Etoposide (VP-16) was dissolved in DMSO and normal saline (DMSO final concentration <1%) and administered via intravenous injection at 10 mg/kg once weekly for 4 weeks. Tumor volume was measured every 3 days, and mice were euthanized to harvest tumors for weight measurement [1] C57BL/6 mice (6-8 weeks old) were intravenously injected with B16 melanoma cells (1×10^5 cells/mouse) to establish lung metastasis model. Mice were treated with Etoposide (VP-16) (12 mg/kg, i.p., once weekly for 3 weeks) or vehicle. After 4 weeks, mice were euthanized, and lung metastatic nodules were counted [8] DBA/2 mice (6 weeks old) were intraperitoneally injected with P388 leukemia cells (1×10^6 cells/mouse). Twenty-four hours later, mice were treated with Etoposide (VP-16) (15 mg/kg, i.p., every other day for 5 days) or vehicle. Survival time was recorded for 30 days [7] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Absorbed well, time to peak plasma concentration is 1-1.5 hrs. Mean bioavailability is 50% (range of 25% - 75%). Cmax and AUC values for orally administered etoposide capsules display intra- and inter-subject variability. There is no evidence of first-pass effect for etoposide. Etoposide is cleared by both renal and nonrenal processes, i.e., metabolism and biliary excretion. Glucuronide and/or sulfate conjugates of etoposide are also excreted in human urine. Biliary excretion of unchanged drug and/or metabolites is an important route of etoposide elimination as fecal recovery of radioactivity is 44% of the intravenous dose. 56% of the dose was in the urine, 45% of which was excreted as etoposide. The disposition of etoposide is a biphasic process with a distribution half-life of 1.5 hours. It does not cross into cerebrospinal fluid well. Volume of distribution, steady state = 18 - 29 L. Total body clearance = 33 - 48 mL/min [IV administration, adults] Mean renal clearance = 7 - 10 mL/min/m^2 Excretion of etoposide in breast milk was demonstrated in a woman with acute promyelocytic leukemia receiving daily doses of 80 mg/sq m (route not stated). Peak concentrations of 0.6 to 0.8 ug/mL were measured immediately after dosing but had decreased to undetectable levels by 24 hr. Thirty minutes after intravenous administration of etoposide to rats, the highest concentrations were found in the liver, kidneys and small intestine. By 24 hr after the dose, the tissue concentrations were negligible. After intravenous infusion (5 min) of etoposide phosphate to beagle dogs at doses of 57-461 mg/sq m, a dose-proportional increase was seen in the maximal plasma concentration and AUC for etoposide. The total plasma clearance rate (342-435 mL/min per sq m) and the distribution volume (22-27 L/sq m) were not dose-dependent. The peak plasma concentration occurred at the end of the infusion of etoposide phosphate, indicating rapid conversion of the pro-drug to etoposide. Less than 4% of a dose was recovered in the bile after 48 hr in patients with biliary drainage tubes. The fecal recovery of radiolabel after intravenous administration of 3(H)etoposide (130-290 mg/sq m) was variable, representing 0-16% of dose, but the collections were known to be incomplete because of fecal retention and other difficulties associated with the poor general condition of many of the patients). In a study reported as an abstract in four patients with small-cell lung cancer given 14(C)-glucopyranoside etoposide, 56% of the radiolabel was recovered in urine and 44% in feces over five days, for a total recovery of 100 +/- 6%. For more Absorption, Distribution and Excretion (Complete) data for ETOPOSIDE (18 total), please visit the HSDB record page. Metabolism / Metabolites Primarily hepatic (through O-demethylation via the CYP450 3A4 isoenzyme pathway) with 40% excreted unchanged in the urine. Etoposide also undergoes glutathione and glucuronide conjugation which are catalyzed by GSTT1/GSTP1 and UGT1A1, respectively. Prostaglandin synthases are also responsible for the conversion of etoposide to O-demethylated metabolites (quinone). The proposed hydroxy acid metabolite of etoposide, formed by opening of the lactone ring, has been detected in human urine, but only at low concentrations, accounting for 0.2-2.2% of the administered dose. The major urinary metabolite of etoposide in humans is reported to be the glucuronide conjugate. Although urinary glucuronide and/or sulfate conjugates were reported to account for 5-22% of an intravenous dose of etoposide, other studies suggest that the glucuronide predominates. Etoposide glucuronide in the urine of treated patients accounted for 8-17% of a dose of 0.5-3.5 g/sq m etoposide and 29% of a dose of 100-800 mg/sq m etoposide, with no other metabolites other than etoposide glucuronide detected in the latter study. In patients with renal or liver impairment given somewhat lower doses of 70-150 mg/sq m, 3-17% of the dose was excreted in the urine within 72 hr as etoposide glucuronide. Etoposide appears to be metabolized principally at the D ring to produce the resulting hydroxy acid (probably the trans-hydroxy acid); this metabolite appears to be pharmacologically inactive. The picrolactone isomer of etoposide has been detected in two concentrations in the plasma and urine of some patients but not in others. The aglycone of etoposide and/or its conjugates have not been detected to date in patients receiving the drug. In vitro, the picrolactone isomer and aglycone of etoposide have minimal cytotoxic activity. Generally, few or no etoposide metabolites have been detected in plasma. Etoposide is administered as the trans-lactone, but cis-etoposide can also be detected in human urine. This might be a storage phenomenon, since isomerization sometimes occurs during freezing of plasma samples under slightly basic conditions. The cis isomer accounts for < 1% of the dose. The catechol metabolite has also been reported in patients receiving 600 mg/sq m etoposide, with an AUC of around 2.5% that of etoposide. In patients given 90 mg/sq m etoposide, the catechol metabolite represented 1.4-7.1% of the urinary etoposide and < 2% of the administered dose. In rat liver homogenates, liver microsomes and in rats in vivo, etoposide was extensively metabolized to only one major metabolite, which was not formally identified. In perfused isolated rat liver incubated with etoposide, the total recovery in bile was 60-85%, with roughly equal amounts of etoposide and two glucuronide metabolites, confirmed as glucuronide species by liquid chromatography and mass spectrometry. After intravenous injection of 3(H)etoposide to rabbits, the total urinary excretion of radiolabel was 30% after five days, with very little thereafter. A single glucuronide metabolite was identified in rabbit urine, which was present in larger amounts than etoposide. No hydroxy acid was identified in either species. Primarily hepatic (through O-demethylation via the CYP450 3A4 isoenzyme pathway) with 40% excreted unchanged in the urine. Etoposide also undergoes glutathione and glucuronide conjugation which are catalyzed by GSTT1/GSTP1 and UGT1A1, respectively. Prostaglandin synthases are also responsible for the conversion of etoposide to O-demethylated metabolites (quinone). Route of Elimination: Etoposide is cleared by both renal and nonrenal processes, i.e., metabolism and biliary excretion. Glucuronide and/or sulfate conjugates of etoposide are also excreted in human urine. Biliary excretion of unchanged drug and/or metabolites is an important route of etoposide elimination as fecal recovery of radioactivity is 44% of the intravenous dose. 56% of the dose was in the urine, 45% of which was excreted as etoposide. Half Life: 4-11 hours Biological Half-Life 4-11 hours ... In adults with normal renal and hepatic function, the half-life of etoposide averages 0.6-2 hours ... in the initial phase and 5.3-10.8 hours ... in the terminal phase. In one adult with impaired hepatic function, the terminal elimination half-life was reportedly 78 hours. In children with normal renal and hepatic function, the half-life of etoposide averages 0.6-1.4 hours in the initial phase and 3-5.8 hours in the terminal phase. ... Elimination half-life of 3 to 7 hr in children and 4 to 8 hr in adults. Etoposide (VP-16) has a terminal half-life (t1/2) of 7.5 hours in humans after intravenous administration (60 mg/m²) [1] Etoposide (VP-16) shows low oral bioavailability (15-30%) in humans due to first-pass metabolism [4] Etoposide (VP-16) has a volume of distribution (Vd) of 0.2-0.4 L/kg in humans and 1.0 L/kg in rats [1,5] Etoposide (VP-16) is metabolized in the liver via cytochrome P450 (CYP3A4/5) and excreted primarily in urine (40-60%) and feces (10-20%) [4] |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Etoposide inhibits DNA topoisomerase II, thereby inhibiting DNA re-ligation. This causes critical errors in DNA synthesis at the premitotic stage of cell division and can lead to apoptosis of the cancer cell. Etoposide is cell cycle dependent and phase specific, affecting mainly the S and G2 phases of cell division. Inhibition of the topoisomerase II alpha isoform results in the anti-tumour activity of etoposide. The drug is also capable of inhibiting the beta isoform but inhibition of this target is not associated with the anti-tumour activity. It is instead associated with the carcinogenic effect. Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Most sources consider breastfeeding to be contraindicated during maternal antineoplastic drug therapy. It might be possible to breastfeed safely during intermittent therapy with etoposide after an appropriate period of breastfeeding abstinence. A period of at least 24 hours is required after a dose of 80 mg/sq. m. or less. Others have suggested an abstinence period of 72 hours after etoposide use. Chemotherapy may adversely affect the normal microbiome and chemical makeup of breastmilk. Women who receive chemotherapy during pregnancy are more likely to have difficulty nursing their infant. ◉ Effects in Breastfed Infants One mother received with 5 daily doses of etoposide 80 mg/sq. m. and cytarabine 170 mg/sq. m. intravenously as well as 3 daily doses of 6 mg/sq. m. of mitoxantrone intravenously. She resumed breastfeeding her infant 3 weeks after the third dose of mitoxantrone at a time when mitoxantrone was still detectable in milk. The infant had no apparent abnormalities at 16 months of age. ◉ Effects on Lactation and Breastmilk A telephone follow-up study was conducted on 74 women who received cancer chemotherapy at one center during the second or third trimester of pregnancy to determine if they were successful at breastfeeding postpartum. Only 34% of the women were able to exclusively breastfeed their infants, and 66% of the women reported experiencing breastfeeding difficulties. This was in comparison to a 91% breastfeeding success rate in 22 other mothers diagnosed during pregnancy, but not treated with chemotherapy. Other statistically significant correlations included: 1. mothers with breastfeeding difficulties had an average of 5.5 cycles of chemotherapy compared with 3.8 cycles among mothers who had no difficulties; and 2. mothers with breastfeeding difficulties received their first cycle of chemotherapy on average 3.4 weeks earlier in pregnancy. Of the 9 women who received a taxane-containing regimen, 7 had breastfeeding difficulties. Protein Binding 97% protein bound. Interactions Additive bone marrow depression may occur; dosage reduction may be required when two or more bone marrow depressants, including radiation, are used concurrently or consecutively. Multidrug resistance is one of the mechanisms of resistance to multiple cytotoxic drugs and is mediated by the expression of a membrane pump called the P-glycoprotein. Nifedipine is one of the calcium channel blocking agents which reverses multidrug resistance in vitro. Fifteen patients with various malignancies received nifedipine at three dose levels: 40 mg, 60 mg and 80 mg orally twice daily for 6 days. Etoposide was administered intravenously on day 2 in a dose of 150-250 mg/sq m and orally 150-300 mg twice daily on days 3 and 4. Cardiovascular effects of nifedipine were dose limiting and the maximum tolerated dose was 60 mg twice a day. Mean area under the plasma concentration curve and plasma half-life of nifedipine and its major metabolite MI at the highest dose level were 7.87 uM.hr, 7.97 hr and 4.97 uM.hr, 14.0 hr respectively. Nifedipine did not interfere with the pharmacokinetics of etoposide. Dipyridamole has chemical characteristics similar to other known modulators of etoposide, doxorubicin, and vinblastine sensitivity. When compared to verapamil, dipyridamole was as efficacious but twice as potent in its synergistic enhancement of etoposide sensitivity. These results demonstrate that dipyridamole can markedly increase the cytotoxicity of etoposide, doxorubicin, and vinblastine and suggest possible clinical applications. Enhanced antineoplastic action of etoposide in the presence of cyclosporin A, was investigated in several in vitro and in vivo tumor systems. Macromolecular DNA damage induced by etoposide at drug levels comparable to plasma area under the curve values achieved in patients was increased not only in leukemic peripheral blood cells from patients but also in mononuclear peripheral blood cells from a healthy donor. Intracellular retention of radioactivity from (3)H etoposide was increased by a factor of 1.5 at the most in the presence of cyclosporin A. The cytotoxicity of etoposide and adriamycin to L 1210 leukemic cells was clearly enhanced, whereas cyclosporin A had no effect on the action of cisplatin or ionizing irradiation. At cyclosporin A blood levels not exceeding 1.44 ug/ml, increased tumor inhibition of etoposide was observed in a human embryonal cancer xenograft, but there was also higher lethality in normal mice. With respect to chemosensitization the effects of cyclosporin A resemble those of calcium channel blockers or anticalmodulin agents. In contrast to calcium channel blockers, however, adequate plasma levels of cyclosporin A can well be achieved in patients. For more Interactions (Complete) data for ETOPOSIDE (7 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Mouse IV 118 mg/kg bw LD50 Rat IV 68 mg/kg bw LD50 Rabbit IV > 80 mg/kg bw LD50 Mouse ip 108 mg/kg bw Etoposide (VP-16) has a plasma protein binding rate of 97% in human plasma [4] Etoposide (VP-16) induced myelosuppression in vitro: human bone marrow progenitor cells showed 50% inhibition of colony formation at 0.1 μM [6] In rats treated with Etoposide (VP-16) (20 mg/kg, i.v., weekly for 3 weeks), mild elevation of serum ALT/AST (1.2-fold) was observed, with no significant renal toxicity (BUN/Cr unchanged) [5] Etoposide (VP-16) (in vitro concentration >10 μM) caused gastrointestinal epithelial cell damage, with cell viability reduced by 40% [8] Etoposide (VP-16) has an intravenous LD50 of 200 mg/kg in mice and 150 mg/kg in rats [7] |
| 参考文献 |
[1]. J Natl Cancer Inst . 1988 Dec 7;80(19):1526-33. [2]. J Dermatol Sci . 2000 Nov;24(2):126-33. [3]. Cancer Res . 1983 Apr;43(4):1592-7. [4]. Cancer Chemother Pharmacol . 2001 Oct;48(4):327-32. [5]. Cancer Chemother Pharmacol . 1998;41(2):93-7. [6]. Cancer . 1998 Dec 1;83(11):2400-7. [7]. Gan To Kagaku Ryoho . 1991 Jun;18(7):1155-61. |
| 其他信息 |
Therapeutic Uses
Antineoplastic Agents, Phytogenic; Nucleic Acid Synthesis Inhibitors Etoposide injection is indicated, in combination with other antineoplastics, for first-line treatment of testicular tumors (Evidence rating: 1A). /Included in US product labeling/ Etoposide is indicated in combination with other agents as first-line treatment of small cell lung carcinoma. /Included in US product labeling/ Etoposide also is indicated, alone and in combination with other agents, for treatment of Hodgkin's and non-Hodgkin\"s lymphomas and acute nonlymphocytic (myelocytic) leukemia. /NOT included in US product labeling/ For more Therapeutic Uses (Complete) data for ETOPOSIDE (13 total), please visit the HSDB record page. Drug Warnings The major and dose-limiting adverse effect of etoposide is hematologic toxicity. Myelosuppression, which is dose related, is manifested mainly by leukopenia (principally granulocytopenia). Myelosuppression resulting in death has been reported in patients receiving etoposide. Thrombocytopenia occurs less frequently, and anemia may also occur; pancytopenia has occurred in some patients. Myelosuppression apparently is not cumulative but may be more severe in patients previously treated with other antineoplastic agents or radiation therapy. Leukopenia has reportedly occurred in 60-91% of patients receiving etoposide and was severe (leukocyte count less than 1000/cu mm) in 3-17% of patients. Neutropenia (less than 2000 cu mm) occurred in 88% of patients treated with etoposide phosphate; severe neutropenia has reportedly occurred in 22-41% of patients receiving the drug and was severe (platelet count less than 50,000/cu mm) in 1-20% of patients. Anemia has occurred in up to 33% of patients receiving etoposide. Anemia (hemoglobin less than 11 g/dL) occurred in 72% of patients treated with etoposide phosphate; severe anemia (hemoglobin less than 8 g/dL) occurred in 19% of patients treated. Granulocyte and platelet nadirs usually occur within 7-14 and 9-16 days, respectively, after administration of etoposide, and within 12-19 and 10-15 days, respectively, after administration of etoposide phosphate; leukocyte nadir has been reported to occur within 15-22 days after administration of etoposide, phosphate. Bone marrow recovery is usually complete within 20 days after administration, but may occasionally require longer periods. Fever and infection have been reported in patients with drug-induced neutropenia. Pregnancy risk category: D /POSITIVE EVIDENCE OF RISK. Studies in humans, or investigational or post-marketing data, have demonstrated fetal risk. Nevertheless, potential benefits from the use of the drug may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective./ Reversible alopecia, sometimes progressing to complete baldness, has occurred in 8-66% of patients receiving etoposide. The degree of alopecia may be dose related. Stevens-Johnson syndrome has been reported infrequently in patients receiving etoposide. Rash, pigmentation, urticaria, and severe pruritus have occurred infrequently, and cutaneous radiation-recall reactions associated with etoposide have been reported. ... Anaphylactoid reactions consisting principally of chills, rigors, diaphoresis, pruritus, loss of consciousness, nausea, vomiting, fever, bronchospasm, dyspnea, tachycardia, hypertension, and/or hypotension have occurred during or immediately after administration of etoposide or etoposide phosphate in 0.7-3% of patients receiving the drug. Other manifestations have included flushing, rash, substernal chest pain, lacrimation, sneezing, coryza, throat pain, back pain, generalized body pain, abdominal cramps, and auditory impairment. For more Drug Warnings (Complete) data for ETOPOSIDE (24 total), please visit the HSDB record page. Pharmacodynamics Etoposide is an antineoplastic agent and an epipodophyllotoxin (a semisynthetic derivative of the podophyllotoxins). It inhibits DNA topoisomerase II, thereby ultimately inhibiting DNA synthesis. Etoposide is cell cycle dependent and phase specific, affecting mainly the S and G2 phases. Two different dose-dependent responses are seen. At high concentrations (10 µg/mL or more), lysis of cells entering mitosis is observed. At low concentrations (0.3 to 10 µg/mL), cells are inhibited from entering prophase. It does not interfere with microtubular assembly. The predominant macromolecular effect of etoposide appears to be the induction of DNA strand breaks by an interaction with DNA-topoisomerase II or the formation of free radicals. Etoposide (VP-16) is a semisynthetic derivative of podophyllotoxin, a natural compound isolated from Podophyllum peltatum [3] Etoposide (VP-16) exerts antitumor effects by stabilizing the Topo II-DNA cleavage complex, preventing DNA religation and leading to irreversible DNA double-strand breaks, cell cycle arrest (G2/M phase), and apoptosis [3,6] Etoposide (VP-16) is FDA-approved for the treatment of small cell lung cancer, testicular cancer, ovarian cancer, and Hodgkin's lymphoma [1,6] Etoposide (VP-16) is commonly used in combination chemotherapy regimens due to its synergistic activity with cisplatin, carboplatin, and other chemotherapeutic agents [9] Etoposide (VP-16) resistance may occur via downregulation of Topo IIα expression or increased drug efflux by ABC transporters (e.g., P-glycoprotein) [4] |
| 分子式 |
C29H32O13
|
|---|---|
| 分子量 |
588.56
|
| 精确质量 |
588.184
|
| 元素分析 |
C, 59.18; H, 5.48; O, 35.34
|
| CAS号 |
33419-42-0
|
| 相关CAS号 |
117091-64-2
|
| PubChem CID |
36462
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.6±0.1 g/cm3
|
| 沸点 |
798.1±60.0 °C at 760 mmHg
|
| 熔点 |
236-251ºC
|
| 闪点 |
263.6±26.4 °C
|
| 蒸汽压 |
0.0±3.0 mmHg at 25°C
|
| 折射率 |
1.662
|
| LogP |
0.3
|
| tPSA |
160.83
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
13
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
42
|
| 分子复杂度/Complexity |
969
|
| 定义原子立体中心数目 |
10
|
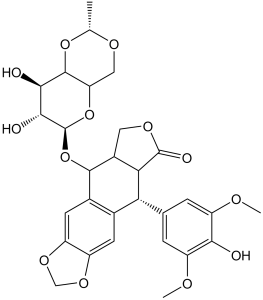
| SMILES |
O=C1OC[C@]2([H])[C@H](O[C@H]3[C@@H]([C@H]([C@@H]4O[C@H](C)OC[C@H]4O3)O)O)C5=C(C=C6OCOC6=C5)[C@@H](C7=CC(OC)=C(O)C(OC)=C7)[C@]21[H]
|
| InChi Key |
VJJPUSNTGOMMGY-MRVIYFEKSA-N
|
| InChi Code |
InChI=1S/C29H32O13/c1-11-36-9-20-27(40-11)24(31)25(32)29(41-20)42-26-14-7-17-16(38-10-39-17)6-13(14)21(22-15(26)8-37-28(22)33)12-4-18(34-2)23(30)19(5-12)35-3/h4-7,11,15,20-22,24-27,29-32H,8-10H2,1-3H3/t11-,15+,20-,21-,22+,24-,25-,26-,27-,29+/m1/s1
|
| 化学名 |
(5S,5aR,8aR,9R)-5-[[(2R,4aR,6R,7R,8R,8aS)-7,8-dihydroxy-2-methyl-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxin-6-yl]oxy]-9-(4-hydroxy-3,5-dimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-[2]benzofuro[6,5-f][1,3]benzodioxol-8-one
|
| 别名 |
Demethyl Epipodophyllotoxin; Ethylidine Glucoside; epipodophyllotoxin; trans-Etoposide; (-)-Etoposide; Lastet; Zuyeyidal; US brand names: Toposar; VePesid. Foreign brand name: Lastet. Abbreviation: EPEG Code names: VP16; VP16213;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.25 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.25 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.25 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.5 mg/mL (4.25 mM) (饱和度未知) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 5 中的溶解度: ≥ 0.5 mg/mL (0.85 mM) (饱和度未知) in 1% DMSO 99% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 6 中的溶解度: 30% Propylene glycol , 5% Tween 80 , 65% D5W: 30 mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6991 mL | 8.4953 mL | 16.9906 mL | |
| 5 mM | 0.3398 mL | 1.6991 mL | 3.3981 mL | |
| 10 mM | 0.1699 mL | 0.8495 mL | 1.6991 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
A Study to Compare Standard Therapy to Treat Hodgkin Lymphoma to the Use of Two Drugs, Brentuximab Vedotin and Nivolumab
CTID: NCT05675410
Phase: Phase 3 Status: Recruiting
Date: 2024-12-02