| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 500mg |
|
||
| Other Sizes |
|

| 靶点 |
Fluoxetine exerts pharmacological effects by targeting the serotonin transporter (SERT), inhibiting the reuptake of serotonin (5-HT) into presynaptic neurons[1]
Fluoxetine modulates the norepinephrine (NE) and dopamine (DA) systems by indirectly increasing extracellular levels of NE and DA in the prefrontal cortex, with the effect mediated through interaction with SERT (no direct binding to NE/DA transporters reported)[5] Fluoxetine synergistically enhances the effects of antipsychotic agents (e.g., olanzapine) on NE and DA release in the prefrontal cortex, with the synergistic effect dependent on its SERT-inhibiting activity[6] |
|---|---|
| 体外研究 (In Vitro) |
在海马细胞中,氟西汀可防止不可避免的休克 (IS) 下调细胞增殖 [1]。氟西汀促进成年大鼠海马齿状回新细胞的生长。在前边缘皮质中,氟西汀还可以增加增殖细胞的数量[2]。服用氟西汀后,处于未成熟状态的神经元成熟得更快。在齿状回,氟西汀可改善神经发生依赖性长时程增强 (LTP) [3]。在前额皮质中,氟西汀增加细胞外去甲肾上腺素和多巴胺的水平,但不增加西酞普兰、氟伏沙明、帕罗西汀或舍曲林的水平。急性全身给药后,氟西汀会导致细胞外多巴胺和去甲肾上腺素浓度强烈且持久的升高[4]。
|
| 体内研究 (In Vivo) |
在暴露于不可避免的休克的成年雄性 Sprague-Dawley 大鼠中,氟西汀治疗也扭转了逃避潜伏期的缺点 [1]。在齿状回中,氟西汀 (5 mg/kg) 本身可促进细胞生长。当氟西汀5 mg/kg和奥氮平同时给药时,与对照组相比,BrdU阳性细胞的数量显着增加[2]。随着氟西汀和奥氮平的联合使用,细胞外多巴胺 ([DA](ex)) 和去甲肾上腺素 ([NE](ex)) 水平显着且稳定地增加,分别达到基线的 361% 和 272%。当单独使用药物时,远高于基线[5]。
在经历不可逃避应激(会降低海马细胞增殖)的成年大鼠中,慢性给予Fluoxetine(氟西汀)可逆转应激诱导的海马细胞增殖减少,表现为齿状回中溴脱氧尿苷(BrdU)标记的细胞数量增加[1] 在暴露于产前应激(会增加产后疾病症状)的大鼠后代中,产后给予Fluoxetine(氟西汀)可预防疾病相关行为(如社交互动减少、焦虑样行为增加)的加剧,并使应激诱导的神经化学变化恢复正常[2] 对成年大鼠慢性给予Fluoxetine(氟西汀)(10 mg/kg/天,持续21天),可显著增加海马齿状回和前额叶皮层的细胞增殖,BrdU标记细胞数量较溶剂对照组增加约50%[3] 成年大鼠慢性给予Fluoxetine(氟西汀)(10 mg/kg/天,持续28天)可促进成年海马颗粒细胞的成熟:增加树突棘数量、增强突触可塑性(通过长时程增强LTP检测)、提高BrdU标记成熟神经元的存活率[4] 对成年大鼠急性给予Fluoxetine(氟西汀)(10 mg/kg,腹腔注射),可显著增加前额叶皮层NE和DA的胞外水平(分别达到基线的约300%和200%),而其他选择性5-羟色胺再摄取抑制剂(SSRI,如帕罗西汀、舍曲林)无此效应[5] 大鼠联合给予Fluoxetine(氟西汀)(10 mg/kg,腹腔注射)与奥氮平(1 mg/kg,腹腔注射),可协同增加前额叶皮层NE和DA释放(NE:基线的约450%;DA:基线的约350%),效应强于单独使用任一药物[6] |
| 酶活实验 |
前额叶皮层胞外NE和DA检测(微透析法):在麻醉大鼠前额叶皮层立体定位植入微透析探针;恢复24小时后,以恒定流速(1 μL/分钟)灌注人工脑脊液(aCSF);在给予Fluoxetine(氟西汀)前后每20分钟收集透析液;通过高效液相色谱(HPLC)结合电化学检测定量透析液中NE和DA的水平[5]
协同NE/DA释放检测(微透析法):微透析操作同上;大鼠在给予Fluoxetine(氟西汀)前30分钟预处理抗精神病药物(如奥氮平);连续收集透析液4小时,通过HPLC-电化学检测分析NE/DA水平,评估协同效应[6] |
| 细胞实验 |
海马细胞增殖检测(BrdU标记法):成年大鼠每日腹腔注射BrdU(50 mg/kg)两次,连续3天以标记增殖细胞;Fluoxetine(氟西汀)处理后,大鼠安乐死,脑组织用多聚甲醛固定;制备30 μm冠状脑切片,通过免疫组化(抗BrdU一抗和荧光二抗孵育)检测BrdU标记细胞;在荧光显微镜下计数海马齿状回中的BrdU阳性细胞[1]
海马颗粒细胞成熟检测:慢性Fluoxetine(氟西汀)处理和BrdU标记后,脑切片用抗BrdU(识别成年新生细胞)与抗双皮质素(DCX,未成熟神经元标志物)或抗NeuN(成熟神经元标志物)抗体共染色;定量BrdU/DCX阳性(未成熟)和BrdU/NeuN阳性(成熟)细胞的百分比,评估神经元成熟度[4] 突触可塑性检测(LTP记录):慢性Fluoxetine(氟西汀)处理后,大鼠麻醉,在穿通通路放置刺激电极,在海马齿状回放置记录电极;通过穿通通路高频刺激(HFS)诱导LTP;记录2小时场兴奋性突触后电位(fEPSP),测定LTP幅度[4] |
| 动物实验 |
Inescapable stress model: Adult male rats were subjected to inescapable footshock (1.6 mA, 10 s duration, 60 shocks) once daily for 7 days; Fluoxetine was dissolved in saline and administered i.p. at 10 mg/kg/day for 21 days (starting 3 days before stress exposure); vehicle controls received saline injections[1]
Prenatal stress model: Pregnant rats were exposed to restraint stress (45 min/day, 3 times/day) from gestational day 12 to 18; offspring rats received Fluoxetine (5 mg/kg/day) via oral gavage from postnatal day 21 to 42; vehicle controls received water via gavage[2] Chronic administration model: Adult male rats were administered Fluoxetine (10 mg/kg/day) or vehicle via i.p. injection once daily for 21 days; BrdU was injected during the last 3 days of treatment to label proliferating cells[3] Neuronal maturation model: Adult male rats were given Fluoxetine (10 mg/kg/day) via i.p. injection once daily for 28 days; BrdU was injected on days 1-3 of treatment to label newly born cells[4] Neurotransmitter release model: Adult male rats were administered Fluoxetine (10 mg/kg) or other SSRIs (paroxetine, 10 mg/kg; sertraline, 10 mg/kg) via i.p. injection; microdialysis was performed 30 min after injection to measure NE/DA levels[5] Drug combination model: Adult male rats were injected i.p. with olanzapine (1 mg/kg) or other antipsychotics (risperidone, 0.5 mg/kg) 30 min before Fluoxetine (10 mg/kg, i.p.) administration; microdialysis was performed to measure prefrontal cortex NE/DA release[6] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The oral bioavailability of fluoxetine is <90% as a result of hepatic first pass metabolism. In a bioequivalence study, the Cmax of fluoxetine 20 mg for the established reference formulation was 11.754 ng/mL while the Cmax for the proposed generic formulation was 11.786 ng/ml. Fluoxetine is very lipophilic and highly plasma protein bound, allowing the drug and it's active metabolite, norfluoxetine, to be distributed to the brain. Fluoxetine is primarily eliminated in the urine. The volume of distribution of fluoxetine and it's metabolite varies between 20 to 42 L/kg. The clearance value of fluoxetine in healthy patients is reported to be 9.6 ml/min/kg. Metabolism / Metabolites Fluoxetine is metabolized to norfluoxetine by CYP1A2, CYP2B6, CYP2C9, CYP2C19, CYP2D6, CYP3A4, and CYP3A5 upon ingestion. Although all of the mentioned enzymes contribute to N-demethylation of fluoxetine, CYP2D6, CYP2C9 and CYP3A4 appear to be the major contributing enzymes for phase I metabolism. In addition, there is evidence to suggest that CYP2C19 and CYP3A4 mediate O-dealkylation of fluoxetine and norfluoxetine to produce para-trifluoromethylphenol which is subsequently metabolized to hippuric acid. Both fluoxetine and norfluoxetine undergo glucuronidation to facilitate excretion. Notably, both the parent drug and active metabolite inhibit CYP2D6 isozymes, and as a result patients who are being treated with fluoxetine are susceptible to drug interactions. Fluoxetine has known human metabolites that include Norfluoxetine, p-Trifluoromethyl phenol, and (2S,3S,4S,5R)-3,4,5-trihydroxy-6-[methyl-[3-phenyl-3-[4-(trifluoromethyl)phenoxy]propyl]amino]oxane-2-carboxylic acid. Limited data from animal studies suggest that fluoxetine may undergo first-pass metabolism may occur via the liver and/or lungs. Fluoxetine appears to be extensively metabolized, likely in the liver, to norfluoxetine and other metabolites. Norfluoxetine, the principal active metabolite, is formed via N-demethylation of fluoxetine. Norfluoxetine appears to be comparable pharmacologic potency as fluoxetine. Fluoxetine and norfluoxetine both undergo phase II glucuronidation reactions in the liver. It is also thought that fluoxetine and norfluoxetine undergo O-dealkylation to form p-trifluoromethylphenol, which is then subsequently metabolized to hippuric acid. Route of Elimination: The primary route of elimination appears to be hepatic metabolism to inactive metabolites excreted by the kidney. The S-enantiomer is eliminated more slowly and is the predominant enantiomer present at steady state. Half Life: 1-3 days [acute administration]; 4-6 days [chronic administration]; 4-16 days [norfluoxetine, acute and chronic administration]. Biological Half-Life The half life of fluoxetine is significant with the elimination half-life of the parent drug averaging 1-3 days after acute administration, and 4-6 days after chronic administration. Further, the elimination half life of it's active metabolite, norfluoxetine, ranges from 4-16 days after both acute and chronic administration. The half-life of fluoxetine should be considered when switching patients from fluoxetine to another antidepressant since marked accumulation occurs after chronic use. Fluoxetine's long half-life may even be beneficial when discontinuing the drug since the risk of withdrawal is minimized. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Fluoxetine is a cholinesterase or acetylcholinesterase (AChE) inhibitor. A cholinesterase inhibitor (or 'anticholinesterase') suppresses the action of acetylcholinesterase. Because of its essential function, chemicals that interfere with the action of acetylcholinesterase are potent neurotoxins, causing excessive salivation and eye-watering in low doses, followed by muscle spasms and ultimately death. Nerve gases and many substances used in insecticides have been shown to act by binding a serine in the active site of acetylcholine esterase, inhibiting the enzyme completely. Acetylcholine esterase breaks down the neurotransmitter acetylcholine, which is released at nerve and muscle junctions, in order to allow the muscle or organ to relax. The result of acetylcholine esterase inhibition is that acetylcholine builds up and continues to act so that any nerve impulses are continually transmitted and muscle contractions do not stop. Among the most common acetylcholinesterase inhibitors are phosphorus-based compounds, which are designed to bind to the active site of the enzyme. The structural requirements are a phosphorus atom bearing two lipophilic groups, a leaving group (such as a halide or thiocyanate), and a terminal oxygen. Toxicity Data LD50=284mg/kg (orally in mice). |
| 参考文献 |
|
| 其他信息 |
Pharmacodynamics
Fluoxetine blocks the serotonin reuptake transporter in the presynaptic terminal, which ultimately results in sustained levels of 5-hydroxytryptamine (5-HT) in certain brain areas. However, fluoxetine binds with relatively poor affinity to 5-HT, dopaminergic, adrenergic, cholinergic, muscarinic, and histamine receptors which explains why it has a far more desirable adverse effect profile compared to earlier developed classes of antidepressants such as tricyclic antidepressants. Fluoxetine is a selective serotonin reuptake inhibitor (SSRI) widely used in the treatment of depression and anxiety disorders[1] The ability of Fluoxetine to reverse stress-induced hippocampal hypoproliferation suggests a role in neurogenesis-dependent antidepressant effects[1] Unlike other SSRIs, Fluoxetine uniquely increases prefrontal cortex NE and DA levels, which may contribute to its distinct therapeutic effects (e.g., improved cognitive function) in depression[5] The synergistic effect of Fluoxetine with antipsychotics on NE/DA release provides a pharmacological basis for combination therapy in treatment-resistant depression or schizophrenia[6] Fluoxetine promotes the maturation and synaptic integration of adult-born hippocampal neurons, which may underlie its long-term antidepressant and cognitive-enhancing effects[4] |
| 分子式 |
C17H18NOF3
|
|---|---|
| 分子量 |
309.32612
|
| 精确质量 |
309.134
|
| CAS号 |
54910-89-3
|
| 相关CAS号 |
Fluoxetine hydrochloride;56296-78-7;(S)-Fluoxetine hydrochloride;114247-06-2;(R)-Fluoxetine hydrochloride;114247-09-5
|
| PubChem CID |
3386
|
| 外观&性状 |
Colorless to light yellow liquid
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
395.1±42.0 °C at 760 mmHg
|
| 熔点 |
158ºC
|
| 闪点 |
192.8±27.9 °C
|
| 蒸汽压 |
0.0±0.9 mmHg at 25°C
|
| 折射率 |
1.511
|
| LogP |
4.09
|
| tPSA |
21.26
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
22
|
| 分子复杂度/Complexity |
308
|
| 定义原子立体中心数目 |
0
|
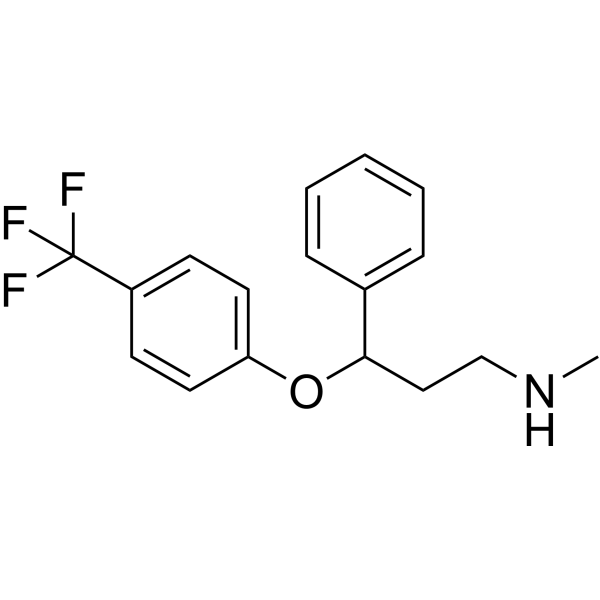
| SMILES |
FC(C1=CC=C(OC(C2=CC=CC=C2)CCNC)C=C1)(F)F
|
| InChi Key |
RTHCYVBBDHJXIQ-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C17H18F3NO/c1-21-12-11-16(13-5-3-2-4-6-13)22-15-9-7-14(8-10-15)17(18,19)20/h2-10,16,21H,11-12H2,1H3
|
| 化学名 |
N-methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy]propan-1-amine
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~323.28 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (6.72 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (6.72 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (6.72 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 10 mg/mL (32.33 mM) in 0.5% CMC-Na/saline water (这些助溶剂从左到右依次添加,逐一添加), 悬浮液; 超声助溶 (<60°C). *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.2328 mL | 16.1640 mL | 32.3279 mL | |
| 5 mM | 0.6466 mL | 3.2328 mL | 6.4656 mL | |
| 10 mM | 0.3233 mL | 1.6164 mL | 3.2328 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Finding Treatments for COVID-19: A Trial of Antiviral Pharmacodynamics in Early Symptomatic COVID-19 (PLATCOV)
CTID: NCT05041907
Phase: Phase 2 Status: Recruiting
Date: 2024-10-28