| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
SSRIs/selective serotonin reuptake inhibitor
|
|---|---|
| 体外研究 (In Vitro) |
基于体外和体内实验,氟伏沙明已被鉴定为一种潜在的抗抑郁药物,几乎完全具有5-羟色胺(5-HT)摄取抑制特性。氟伏沙明可有效抑制血小板和脑突触体对5-羟色胺的摄取。由于膜泵的抑制作用,该化合物防止酪胺衍生物H 75/12和H 77/77消耗5-HT。由于干扰5-HT的神经元再摄取机制,氟伏沙明在大脑中产生5-HT周转减少[3]。
体外活性:氟伏沙明增加大鼠前额皮质和丘脑中的 [5-HT]ex 水平,并且还增加纹状体中的 [DA]ex 水平。 Fluvoxaminemaleate 通过脊髓 5-HT2A/2C 受体作用于受体或 5-HT 神经元,改善触觉异常性疼痛。
Flv/氟伏沙明对SK-N-SH细胞的毒性[4] 使用MTS试验检测Flv对SK-N-SH细胞的毒性。我们使用10、25、50、75或100μg/ml Flv或载体对照治疗SK-N-SH细胞。与载体对照细胞相比,用Flv处理的SK-N-SH细胞显示出80%(25μg/ml)、29%(50μg/ml),19%(75μg/ml)和18%(100μg/ml)的存活率(所有剂量下均为[p<0.001])(图1)。然而,与载体对照相比,用10μg/ml Flv处理的SK-N-SH细胞没有显示出存活率降低(102%)(图1)。基于这些数据,我们在所有后续实验中使用了10μg/ml的Flv。 Flv/氟伏沙明缓解Px诱导的ER应激介导的凋亡[4] 接下来,我们通过监测CHOP、切割的胱天蛋白酶4和切割的胱天蛋白酶3(每种胱天蛋白酶的活性形式),研究了Flv是否可以缓解Px诱导的ER应激介导的SK-N-SH细胞凋亡。与对照细胞相比,用Px处理的细胞中诱导了CHOP、切割的胱天蛋白酶4和切割的胱天蛋白酶3(图2a-c,每次比较时均为[p<0.01]),这与我们之前的报告[19]一致。另一方面,当细胞用Flv预处理,然后用Px/Flv共处理24小时时,与Flv未处理的细胞相比,CHOP、分裂的胱天酶4和分裂的胱天酶3的诱导减轻(图2a-c,分别为p<0.05、p<0.05和p<0.01)。接下来,我们研究了Flv在SK-N-SH细胞中诱导Sig-1R的作用。据报道,Flv不仅是一种强效的Sig-1R激动剂,比其他SSRI具有更强的亲和力[27],而且还是Sig-1R的诱导剂[26]。与未处理的细胞相比,用Flv处理12小时的细胞中诱导了Sig-1R(图2d,p<0.05)。这种诱导持续了至少24小时(图2e,p<0.01)。 Flv/氟伏沙明通过Sig-1R减轻Px诱导的神经毒性[4] 最后,使用MTS测定,我们定量评估了Flv是否可以减轻Px诱导的神经毒性。与蛋白质印迹的结果类似,与Flv未处理的细胞相比,Px处理降低的存活率在Flv预处理的细胞中得到了恢复(图3,p<0.05)。当细胞与Px、Flv和NE100一起孵育时,这种恢复被逆转(图3,p<0.05)。 我们最近报道了Fluvoxamine(Flv)通过诱导sigma-1受体(Sig-1R)缓解ER应激。本研究旨在探讨Flv是否可以在体外减轻Px诱导的神经毒性。SK-N-SH细胞在有或没有10μg/ml Flv的情况下预处理12小时,然后用1μM Px处理24小时,有或没有共存10μg/ml的Flv。为了研究Sig-1R在减轻Px诱导的神经毒性中的作用,加入Sig-1R拮抗剂1μM NE100 24小时。使用MTS存活率测定评估神经毒性,通过评估C/EBP同源蛋白(CHOP)、切割胱天蛋白酶4和切割胱天酶3的表达评估ER应激介导的神经毒性。 用Flv进行预处理显著减轻了SK-N-SH细胞中CHOP、切割胱天蛋白酶4和切割胱天酶3的诱导。同时,Flv预处理显著诱导SK-N-SH细胞中的Sig-1R。此外,Flv处理的细胞的存活率明显高于未处理的细胞,而NE100处理可以逆转这一情况。 我们的结果表明,Flv部分通过诱导Sig-1R减轻了Px诱导的神经毒性。我们的发现应该有助于缓解Px诱导的神经毒性的新方法之一,包括化学脑 [4]。 |
| 体内研究 (In Vivo) |
在 5-HT 溶液中,氟伏沙明 (DU-23000) 可有效抑制脑突触体和突触体。据信,对 5-HT 食物的影响是氟伏沙明对五亚甲基四唑利血平诱导的惊厥阈值降低的单独拮抗作用的原因。当服用快速伏沙明制剂后服用活性利血平样化合物时,与去甲基吡啶和嘧啶的活性相反,在杂质中没有观察到刺激作用[1]。氟伏沙明 (DU-23000) 似乎可以缓解与战斗相关的 PTSD 症状,但不能缓解抑郁症状。我们的研究结果是水分含量高,并且对水分分组没有限制。有必要对氟伏沙明治疗PTSD进行对照研究[2]。当食物与乙醇同时提供时,氟伏沙明 (DU-23000) 减少乙醇自身药物的效果低于单独提供乙醇时的效果(ED50:4.0 (2.7-5.9) 对比 5.1 (4.3-6.0))。当纺锤体能够接触到食物时,对食物的影响是相当的。氟伏沙明在减少乙醇维持的行为方面的有效性取决于乙醇是否与同时计划的食品强化结合使用[3]。
本研究旨在探讨抗抑郁药氟伏沙明治疗战斗相关创伤后应激障碍(PTSD)的疗效。招募了15名患有与战斗有关的创伤后应激障碍且除抑郁症外没有其他精神病诊断的退伍军人参加为期14周的氟伏沙明开放标签研究。患者接受了30天的洗脱期,并在基线时以及每2周至第14周使用临床医生管理的创伤后应激障碍量表(CAPS)、密西西比量表、贝克抑郁量表(BDI)、汉密尔顿抑郁量表。三名患者因副作用过早停止使用氟伏沙明,七名患者在完成14周的试验前撤回了同意书。8名患者完成了至少8周的治疗。氟伏沙明的总日剂量为100-300mg,第14周的平均日剂量为150mg。意向治疗分析显示,CAPS总分、侵入和回避/麻木子量表均有显著改善。CAPS过度觉醒评分没有显著变化。HAM-A评分也显著提高。在意向治疗分析中,密西西比量表、HAM-D或贝克抑郁量表没有明显变化。总之,我们的研究表明,氟伏沙明似乎可以改善与战斗相关的创伤后应激障碍症状,但不能改善抑郁症状。高损耗率和缺乏安慰剂组限制了我们的研究结论。氟伏沙明治疗创伤后应激障碍的对照研究是有必要的。[1] 选择性血清素再摄取抑制剂氟伏沙明在较低剂量下降低了对乙醇的反应,而不是在单独的成分或单独的大鼠组中对食物的反应。然而,当两者同时可用且每次分娩的产量相等时,这种明显的选择性会逆转,食物维持行为比乙醇维持行为对氟伏沙明的降速作用更敏感。在这里,我们进一步研究了同时获得食物和乙醇对氟伏沙明效力的影响。在食物和乙醇同时可用且反应率相等的条件下[食物和乙醇的平均可变间隔(VI)分别为405秒和14秒],以及在食物输送密度增加的情况下(食物的平均VI为60秒,乙醇的VI为14秒),评估了氟伏沙明(5.6-17.8 mg/kg)的效力。当只有乙醇可用时(食物灭绝,乙醇的平均VI为14秒)和在多个VI下(食物和乙醇的VI为30秒)也测定了氟伏沙明的效力,其中食物或乙醇是每个成分中唯一可用的程序化强化。当食物同时可用时{ED50[95%置信区间(CL):8.2(6.5-10.3)和10.7(7.9-14.4)]},氟伏沙明在减少乙醇自我给药方面的效果不如单独使用乙醇时[ED50:4.0(2.7-5.9)和5.1(4.3-6.0)]。在每种食物供应条件下,对食物的影响都是相似的。结果表明,氟伏沙明在减少乙醇维持行为方面的效力取决于乙醇是单独可用还是在同时进行的食品强化中可用。[2] 1.在体外和体内实验的基础上,氟伏沙明被认为是一种潜在的抗抑郁药物,几乎只具有抑制5-羟色胺(5-HT)摄取的特性。2.氟伏沙明能有效抑制血小板和脑突触体对5-ht的摄取。由于膜泵的抑制,该化合物防止酪胺衍生物H 75/12和H 77/77耗尽5-HT。由于干扰了5-HT的神经元再摄取机制,氟伏沙明导致大脑中5-HT的周转减少。5-羟色氨酸(5-HTP)在小鼠体内的作用增强,与帕吉林联合使用,氟伏沙明诱导5-羟色胺样行为效应。3.与三环类抗抑郁药相比,去甲肾上腺素的摄取过程要么不受氟伏沙明的影响,要么仅受到轻微抑制。酪胺衍生物的去甲肾上腺素耗竭作用不受氟伏沙明的影响。利血平的作用,如上睑下垂,只有在非常高剂量的试验化合物下才会受到影响。氟伏沙明对利血平诱导的五亚甲基四唑惊厥阈值降低的拮抗作用可以被认为是由于对5-HT摄取的影响。与去甲基丙咪嗪和丙咪嗪的作用相反,在服用一定剂量的氟伏沙明后给予快速作用的利血平样化合物时,在大鼠体内没有发现刺激作用[3]。 |
| 细胞实验 |
MTS细胞活力测定[4]
使用CellTiter 96单水溶液细胞增殖测定法评估细胞活力。简而言之,将SK-N-SH细胞接种在96孔板中。允许细胞附着24小时。为了评估Flv对SK-N-SH细胞的毒性,用10、25、50、75或100μg/ml Flv在37°C下处理细胞24小时。为了评估Flv对Px诱导的神经毒性的缓解作用,将SK-N-SH细胞用或不用10μg/ml Flv预处理12小时,然后用或不加10μg/ml Flv的1μM Px处理24小时。为了证实Sig-1 R参与对Px诱发的神经毒性缓解作用,用1μM Plx、10μg/ml Flv和1μM NE100孵育SK-N-SH细胞24小时。接下来,向每个孔中加入20μl MTS试剂,并孵育细胞2小时。使用Micro-Plate Reader在490nm处测量光密度。 蛋白质印迹[4] SK-N-SH细胞用或不用10μg/ml Flv预处理12小时,然后在37°C下用或不使用10μg/ml Flv预处理1μM Px 24小时。细胞在Tris缓冲盐水(TBS)中洗涤,收获,并在RIPA缓冲液中用蛋白酶抑制剂混合物 和磷酸酶抑制剂混合物裂解。将裂解物在冰上超声处理三次,每次5秒,然后孵育15分钟。在13000g下离心20分钟后,保留上清液并在SDS样品缓冲液中煮沸。在SDS聚丙烯酰胺凝胶上分离裂解物(10μg),并将其转移到聚偏二氟乙烯(PVDF)膜上。通过在室温下将膜在TBS-T[50 mM Tris–HCl(pH 7.6)、150 mM NaCl和0.1%v/v Tween-20]中的5%w/v脱脂奶粉中孵育1小时来阻断非特异性蛋白质结合。将膜与以下一级抗体在4°C下孵育过夜:抗CHOP(1:1000)、抗caspase 4(1:500)、反caspase 3(1:1000。然后将膜在TBS-T中洗涤三次,持续5分钟。最后,将膜与HRP缀合的抗兔或抗小鼠抗体在室温下孵育60分钟。使用ECL-Plus试剂盒检测蛋白质条带。使用NIH图像J软件对每个条带的强度进行量化。 |
| 动物实验 |
The selective serotonin reuptake inhibitor fluvoxamine reduces responding for ethanol at lower doses than responding for food when each is available in separate components or separate groups of rats. However, when both are available concurrently and deliveries earned per session are equal, this apparent selectivity inverts and food-maintained behavior is more sensitive than ethanol-maintained behavior to rate-decreasing effects of fluvoxamine. Here, we investigated further the impact that concurrent access to both food and ethanol has on the potency of fluvoxamine. Fluvoxamine (5.6-17.8 mg/kg) potency was assessed under conditions in which food and ethanol were available concurrently and response rates were equal [average variable intervals (VIs) 405 and 14 s for food and ethanol, respectively], as well as when density of food delivery was increased (average VI 60 s for food and VI 14 s for ethanol). The potency of fluvoxamine was also determined when only ethanol was available (food extinction and average VI 14 s for ethanol) and under multiple VIs (VI 30 s for food and ethanol) wherein either food or ethanol was the only programmed reinforcement available during each component. Fluvoxamine was less potent at decreasing ethanol self-administration when food was available concurrently {ED50 [95% confidence limit (CL): 8.2 (6.5-10.3) and 10.7 (7.9-14.4)]} versus when ethanol was available in isolation [ED50: 4.0 (2.7-5.9) and 5.1 (4.3-6.0)]. Effects on food were similar under each condition in which food was available. The results demonstrate that the potency of fluvoxamine in reducing ethanol-maintained behavior depends on whether ethanol is available in isolation or in the context of concurrently scheduled food reinforcement[2].
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Well absorbed, bioavailability of fluvoxamine maleate is 53%. Nine metabolites were identified following a 5 mg radio labelled dose of fluvoxamine maleate, constituting approximately 85% of the urinary excretion products of fluvoxamine. The main human metabolite was fluvoxamine acid which, together with its N-acetylated analog, accounted for about 60% of the urinary excretion products. Approximately 2% of fluvoxamine was excreted in urine unchanged. Following a 14C-labelled oral dose of fluvoxamine maleate (5 mg), an average of 94% of drug-related products was recovered in the urine within 71 hours. 25 L/kg. Metabolism / Metabolites Fluvoxamine is metabolized extensively by the liver. Fluvoxamine has known human metabolites that include Fluvoxamino alcohol. Hepatic Route of Elimination: The main human metabolite was fluvoxamine acid which, together with its N-acetylated analog, accounted for about 60% of the urinary excretion products. Approximately 2% of fluvoxamine was excreted in urine unchanged. Following a 14C-labelled oral dose of fluvoxamine maleate (5 mg), an average of 94% of drug-related products was recovered in the urine within 71 hours. Half Life: 15.6 hours Biological Half-Life 15.6 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
The exact mechanism of action of fluvoxamine has not been fully determined, but appears to be linked to its inhibition of CNS neuronal uptake of serotonin. Fluvoxamine blocks the reuptake of serotonin at the serotonin reuptake pump of the neuronal membrane, enhancing the actions of serotonin on 5HT1A autoreceptors. In-vitro studies suggest that fluvoxamine is more potent than clomipramine, fluoxetine, and desipramine as a serotonin-reuptake inhibitor. Studies have also demonstrated that fluvoxamine has virtually no affinity for alpha1- or alpha2-adrenergic, beta-adrenergic, muscarinic, dopamine D2, histamine H1, GABA-benzodiazepine, opiate, 5-HT1, or 5-HT2 receptors. Hepatotoxicity Liver test abnormalities have been reported to occur in up to 1% patients on fluvoxamine, but elevations are usually modest and usually do not require dose modification or discontinuation. A few instances of acute, clinically apparent episodes of liver injury with marked liver enzyme elevations with no or minimal jaundice have been reported in patients on fluvoxamine. The onset of injury was within a few days of starting therapy and the pattern of serum enzyme elevations was hepatocellular or mixed. Autoimmune (autoantibodies) and immunoallergic features (rash, fever, eosinophilia) were not mentioned. Too few cases have been reported to characterize the clinical features of the liver injury in any detail. In large scale analyses of hepatic adverse events due to antidepressants and SSRIs, fluvoxamine is rarely mentioned. Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Limited information indicates that maternal fluvoxamine doses of up to 300 mg daily produce low levels in breastmilk and would not be expected to cause any adverse effects in breastfed infants, especially if the infant is older than 2 months. If the mother requires fluvoxamine, it is not a reason to discontinue breastfeeding. A safety scoring system finds fluvoxamine use to be possible during breastfeeding. One infant was reported to have an elevated serum level of fluvoxamine, but most who have been tested have undetectable serum levels. Another infant developed diarrhea, vomiting and stimulation after maternal initiation of fluvoxamine. A limited amount of long-term follow-up on growth and development has found no adverse effects in breastfed infants. Monitor infants exposed to fluvoxamine through breast milk for diarrhea, vomiting, decreased sleep, and agitation. Mothers taking an SSRI during pregnancy and postpartum may have more difficulty breastfeeding, although this might be a reflection of their disease state. These mothers may need additional breastfeeding support. Breastfed infants exposed to an SSRI during the third trimester of pregnancy have a lower risk of poor neonatal adaptation than formula-fed infants. ◉ Effects in Breastfed Infants One infant whose mother began taking fluvoxamine 100 mg daily 17 weeks postpartum was breastfed from birth to 5 months of age. The medical and nursing staff did not note any adverse effect in the infant during the 10 weeks of observation during maternal hospitalization. The infant had normal Bayley developmental scores at age 4 months and 21 months. No adverse effects were found in 2 infants, a partially breastfed 26-month-old during maternal intake of 150 mg daily, who also had a normal Denver Developmental Score, and an exclusively breastfed 3-week-old during maternal intake of 50 mg daily. Three mothers who took an average fluvoxamine dose of 117 mg once daily breastfed their infants exclusively for 4 months and at least 50% during months 5 and 6. Their infants had 6-month weight gains that were normal according to national growth standards and the mothers reported no abnormal effects in their infants. One study of the side effects of SSRI antidepressants in nursing mothers found no adverse reactions that required medical attention in one infant whose mother was taking fluvoxamine. No specific information on maternal fluvoxamine dosage, extent of breastfeeding or infant age was reported. A woman who was treated chronically with quetiapine 400 mg and fluvoxamine 200 mg daily took the drugs throughout pregnancy and postpartum. She partially breastfed her infant (extent not stated) for 3 months from birth. No adverse events were seen in the infant who developed normally. A cohort of 247 infants exposed to an antidepressant in utero during the third trimester of pregnancy were assessed for poor neonatal adaptation (PNA). Of the 247 infants, 154 developed PNA. Infants who were exclusively given formula had about 3 times the risk of developing PNA as those who were exclusively or partially breastfed. Four of the infants were exposed to low doses of fluvoxamine in utero and none had PNA. A 5-month-old infant developed severe diarrhea (15 times daily), mild vomiting (2 to 3 times daily), agitation and decreased sleep within 2 days after maternal initiation of fluvoxamine 50 mg daily. Symptoms resolved within 24 hours after the mother discontinued the drug and recurred a week later after fluvoxamine was restarted in the mother. Other causes of the gastrointestinal symptoms could not be found. Fluvoxamine was probably the cause of the reaction. The authors speculate that the infant might have abnormal metabolism of the drug that resulted in high serum concentrations. In a retrospective cohort study of 5,079 newborns whose mothers took an SSRI during pregnancy, 1.5% of breastfed newborns had neonatal withdrawal compared with 2.3% among the formula-fed newborns, although this did not reach statistical significance. Breastfed newborns had a reduced risk of transfer to the NICU than formula-fed newborns; however, this finding did not persist in sensitivity analysis. Only one woman in the study was taking paroxetine. ◉ Effects on Lactation and Breastmilk Fluvoxamine has caused increased prolactin levels and galactorrhea in nonpregnant, nonnursing patients. In one case, euprolactinemic gynecomastia and galactorrhea occurred in a 19-year-old man who was also taking risperidone. In a study of cases of hyperprolactinemia and its symptoms (e.g., gynecomastia) reported to a French pharmacovigilance center, fluvoxamine was found to have a 4.5-fold increased risk of causing hyperprolactinemia compared to other drugs. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. In a small prospective study, 8 primiparous women who were taking a serotonin reuptake inhibitor (SRI; 3 taking fluoxetine and 1 each taking citalopram, duloxetine, escitalopram, paroxetine or sertraline) were compared to 423 mothers who were not taking an SRI. Mothers taking an SRI had an onset of milk secretory activation (lactogenesis II) that was delayed by an average of 16.7 hours compared to controls (85.8 hours postpartum in the SRI-treated mothers and 69.1 h in the untreated mothers), which doubled the risk of delayed feeding behavior in the untreated group. However, the delay in lactogenesis II may not be clinically important, since there was no statistically significant difference between the groups in the percentage of mothers experiencing feeding difficulties after day 4 postpartum. A case control study compared the rate of predominant breastfeeding at 2 weeks postpartum in mothers who took an SSRI antidepressant throughout pregnancy and at delivery (n = 167) or an SSRI during pregnancy only (n = 117) to a control group of mothers who took no antidepressants (n = 182). Among the two groups who had taken an SSRI, 33 took citalopram, 18 took escitalopram, 63 took fluoxetine, 2 took fluvoxamine, 78 took paroxetine, and 87 took sertraline. Among the women who took an SSRI, the breastfeeding rate at 2 weeks postpartum was 27% to 33% lower than mother who did not take antidepressants, with no statistical difference in breastfeeding rates between the SSRI-exposed groups. An observational study looked at outcomes of 2859 women who took an antidepressant during the 2 years prior to pregnancy. Compared to women who did not take an antidepressant during pregnancy, mothers who took an antidepressant during all 3 trimesters of pregnancy were 37% less likely to be breastfeeding upon hospital discharge. Mothers who took an antidepressant only during the third trimester were 75% less likely to be breastfeeding at discharge. Those who took an antidepressant only during the first and second trimesters did not have a reduced likelihood of breastfeeding at discharge. The antidepressants used by the mothers were not specified. A retrospective cohort study of hospital electronic medical records from 2001 to 2008 compared women who had been dispensed an antidepressant during late gestation (n = 575; fluvoxamine n = 18) to those who had a psychiatric illness but did not receive an antidepressant (n = 1552) and mothers who did not have a psychiatric diagnosis (n = 30,535). Women who received an antidepressant were 37% less likely to be breastfeeding at discharge than women without a psychiatric diagnosis, but no less likely to be breastfeeding than untreated mothers with a psychiatric diagnosis. In a study of 80,882 Norwegian mother-infant pairs from 1999 to 2008, new postpartum antidepressant use was reported by 392 women and 201 reported that they continued antidepressants from pregnancy. Compared with the unexposed comparison group, late pregnancy antidepressant use was associated with a 7% reduced likelihood of breastfeeding initiation, but with no effect on breastfeeding duration or exclusivity. Compared with the unexposed comparison group, new or restarted antidepressant use was associated with a 63% reduced likelihood of predominant, and a 51% reduced likelihood of any breastfeeding at 6 months, as well as a 2.6-fold increased risk of abrupt breastfeeding discontinuation. Specific antidepressants were not mentioned. Treatment Treatment should consist of those general measures employed in the management of overdosage with any antidepressant. Ensure an adequate airway, oxygenation, and ventilation. Monitor cardiac rhythm and vital signs. General supportive and symptomatic measures are also recommended. Induction of emesis is not recommended. Gastric lavage with a large-bore orogastric tube with appropriate airway protection, if needed, may be indicated if performed soon after ingestion, or in symptomatic patients. Activated charcoal should be administered. Due to the large volume of distribution of this drug, forced diuresis, dialysis, hemoperfusion and exchange transfusion are unlikely to be of benefit. No specific antidotes for fluvoxamine are known. Protein Binding ~77-80% (plasma protein). |
| 参考文献 |
|
| 其他信息 |
Fluvoxamine is an oxime O-ether that is benzene substituted by a (1E)-N-(2-aminoethoxy)-5-methoxypentanimidoyl group at position 1 and a trifluoromethyl group at position 4. It is a selective serotonin reuptake inhibitor that is used for the treatment of obsessive-compulsive disorder. It has a role as an antidepressant, a serotonin uptake inhibitor and an anxiolytic drug. It is a 5-methoxyvalerophenone O-(2-aminoethyl)oxime and a member of (trifluoromethyl)benzenes. It is functionally related to a (trifluoromethyl)benzene.
Fluvoxamine is an antidepressant which functions pharmacologically as a selective serotonin reuptake inhibitor. Though it is in the same class as other SSRI drugs, it is most often used to treat obsessive-compulsive disorder. Fluvoxamine has been in use in clinical practice since 1983 and has a clinical trial database comprised of approximately 35,000 patients. It was launched in the US in December 1994 and in Japan in June 1999. As of the end of 1995, more than 10 million patients worldwide have been treated with fluvoxamine. Fluvoxamine is a Serotonin Reuptake Inhibitor. The mechanism of action of fluvoxamine is as a Serotonin Uptake Inhibitor. Fluvoxamine is a selective serotonin reuptake inhibitor (SSRI) used in the therapy of obsessive-compulsive disorder. Fluvoxamine therapy can be associated with transient asymptomatic elevations in serum aminotransferase levels and has been linked to rare instances of clinically apparent acute liver injury. Fluvoxamine is a 2-aminoethyl oxime ether of aralkylketones, with antidepressant, antiobsessive-compulsive, and anxiolytic properties. Fluvoxamine, chemically unrelated to other selective serotonin reuptake inhibitors, selectively blocks serotonin reuptake by inhibiting the serotonin reuptake pump at the presynaptic neuronal membrane. This increases serotonin levels within the synaptic cleft, prolongs serotonergic transmission and decreased serotonin turnover, thereby leading to antidepressant, anxiolytic and antiobsessive-compulsive effects. Fluvoxamine shows no significant affinity for histaminergic, alpha or beta adrenergic, muscarinic, or dopaminergic receptors in vitro. Fluvoxamine is an antidepressant which functions pharmacologically as a selective serotonin reuptake inhibitor. Though it is in the same class as other SSRI drugs, it is most often used to treat obsessive-compulsive disorder. Fluvoxamine has been in use in clinical practice since 1983 and has a clinical trial database comprised of approximately 35,000 patients. It was launched in the US in December 1994 and in Japan in June 1999. As of the end of 1995, more than 10 million patients worldwide have been treated with fluvoxamine. A selective serotonin reuptake inhibitor that is used in the treatment of DEPRESSION and a variety of ANXIETY DISORDERS. See also: Fluvoxamine Maleate (has salt form); Fluvoxamine, (Z)- (annotation moved to). Drug Indication Indicated predominantly for the management of depression and for Obsessive Compulsive Disorder (OCD). Has also been used in the management of bulimia nervosa. FDA Label Mechanism of Action The exact mechanism of action of fluvoxamine has not been fully determined, but appears to be linked to its inhibition of CNS neuronal uptake of serotonin. Fluvoxamine blocks the reuptake of serotonin at the serotonin reuptake pump of the neuronal membrane, enhancing the actions of serotonin on 5HT1A autoreceptors. Studies have also demonstrated that fluvoxamine has virtually no affinity for α1- or α2-adrenergic, β-adrenergic, muscarinic, dopamine D2, histamine H1, GABA-benzodiazepine, opiate, 5-HT1, or 5-HT2 receptors, despite having an affinity for binding to σ1 receptors. Pharmacodynamics Fluvoxamine, an aralkylketone-derivative agent, is one of a class of antidepressants known as selective serotonin reuptake inhibitors (SSRIs) that differs structurally from other SSRIs. It is used to treat the depression associated with mood disorders. It is also used on occassion in the treatment of body dysmorphic disorder and anxiety. The antidepressant, antiobsessive-compulsive, and antibulimic actions of Fluvoxamine are presumed to be linked to its inhibition of CNS neuronal uptake of serotonin. In vitro studies show that Fluvoxamine is a potent and selective inhibitor of neuronal serotonin reuptake and has only very weak effects on norepinephrine and dopamine neuronal reuptake. Moreover, apart from binding to σ1 receptors, fluvoxamine has no significant affinity for adrenergic (alpha1, alpha2, beta), cholinergic, GABA, dopaminergic, histaminergic, serotonergic (5HT1A, 5HT1B, 5HT2), or benzodiazepine receptors; antagonism of such receptors has been hypothesized to be associated with various anticholinergic, sedative, and cardiovascular effects for other psychotropic drugs. Furthermore, some studies have demonstrated that the chronic administration of Fluvoxamine was found to downregulate brain norepinephrine receptors (as has been observed with other drugs effective in the treatment of major depressive disorder), while others suggest the opposite. |
| 分子式 |
C15H21N2O2F3
|
|---|---|
| 分子量 |
318.33464
|
| 精确质量 |
318.155
|
| 元素分析 |
C, 56.60; H, 6.65; F, 17.90; N, 8.80; O, 10.05
|
| CAS号 |
54739-18-3
|
| 相关CAS号 |
Fluvoxamine maleate;61718-82-9; Fluvoxamine; 54739-18-3; (E)-Fluvoxamine-d4 maleate; 1432075-74-5;
|
| PubChem CID |
5324346
|
| 外观&性状 |
Colorless to light yellow liquid
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
370.6±52.0 °C at 760 mmHg
|
| 熔点 |
120-122.5ºC
|
| 闪点 |
177.9±30.7 °C
|
| 蒸汽压 |
0.0±0.8 mmHg at 25°C
|
| 折射率 |
1.474
|
| LogP |
3.11
|
| tPSA |
56.84
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
22
|
| 分子复杂度/Complexity |
327
|
| 定义原子立体中心数目 |
0
|
| SMILES |
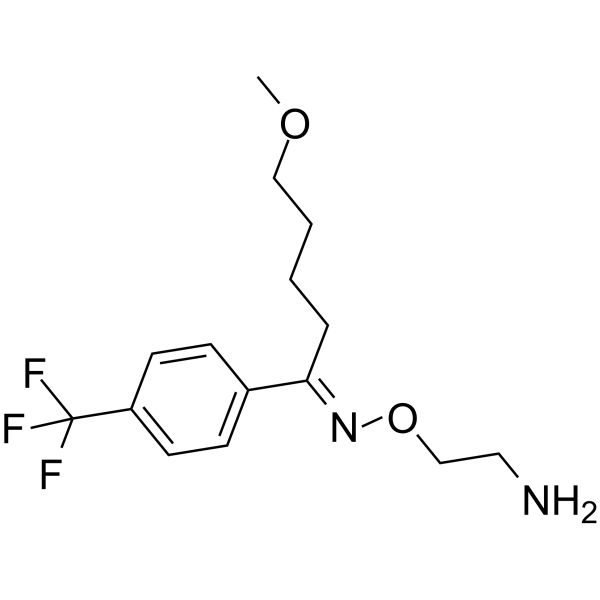
FC(C1=CC=C(/C(CCCCOC)=N/OCCN)C=C1)(F)F
|
| InChi Key |
CJOFXWAVKWHTFT-XSFVSMFZSA-N
|
| InChi Code |
InChI=1S/C15H21F3N2O2/c1-21-10-3-2-4-14(20-22-11-9-19)12-5-7-13(8-6-12)15(16,17)18/h5-8H,2-4,9-11,19H2,1H3/b20-14+
|
| 化学名 |
1-Pentanone, 5-methoxy-1-(4-(trifluoromethyl)phenyl)-, O-(2-aminoethyl)oxime, (E)-
|
| 别名 |
DU-23000; fluvoxamine; 54739-18-3; Fluvoxamina; Fluvoxaminum; Fluvoxaminum [INN-Latin]; Fluvoxamina [INN-Spanish]; UNII-O4L1XPO44W; O4L1XPO44W; DU23000
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~160 mg/mL (~502.62 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (6.53 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.08 mg/mL (6.53 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (6.53 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1414 mL | 15.7070 mL | 31.4139 mL | |
| 5 mM | 0.6283 mL | 3.1414 mL | 6.2828 mL | |
| 10 mM | 0.3141 mL | 1.5707 mL | 3.1414 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04885530 | Active Recruiting |
Drug: Ivermectin Drug: Fluvoxamine Drug: Fluticasone |
Covid19 | Susanna Naggie, MD | June 8, 2021 | Phase 3 |
| NCT04510194 | Active Recruiting |
Drug: Metformin Drug: Placebo Drug: Fluvoxamine |
Covid19 SARS-CoV Infection |
University of Minnesota | January 1, 2021 | Phase 3 |
| NCT04160377 | Recruiting | Drug: Fluvoxamine | Depressive Disorder Endogenous Depression Melancholia |
Lingjiang Li | August 1, 2019 | Phase 2 |
| NCT04963257 | Recruiting | Drug: sertraline fluvoxamine Drug: sertraline |
OCD | Second Affiliated Hospital, School of Medicine, Zhejiang University |
January 1, 2020 | Phase 4 |
| NCT05874037 | Recruiting | Drug: Fluvoxamine | Long COVID | Washington University School of Medicine |
May 15, 2023 | Phase 2 Phase 3 |