| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
| 靶点 |
Spleen Tyrosine Kinase (Syk) (active metabolite R406, recombinant human Syk, IC50 = 41 nM); parent drug Fostamatinib Disodium has no direct Syk inhibitory activity, requiring hydrolysis to R406 for efficacy [1][2]
- R406 (metabolite) exhibits >50-fold selectivity over off-target kinases: Lyn (IC50 = 2200 nM), Src (IC50 = 3100 nM), JAK2 (IC50 = 4500 nM) [2] - Confirmed Syk as primary target of R406 (rheumatoid arthritis synoviocyte model; consistent with [2]’s selectivity) [3] |
|---|---|
| 体外研究 (In Vitro) |
R935788是R406的亚甲基磷酸酯前药,在体内可快速转化为R406。 R406(R935788 的体外活性形式)选择性抑制 Syk 依赖性信号传导,EC50 值范围为 33 nM 至 171 nM,比不同细胞中的 Syk 独立途径更有效。 R406 抑制多种弥漫性大 B 细胞淋巴瘤 (DLBCL) 细胞系的细胞增殖,EC50 值范围为 0.8 μM 至 8.1 μM。 R406 治疗不仅在高 (TCL-002) 细胞中而且在磷酸化 Syk 水平低的细胞中降低 BLNK、Akt、糖原合酶激酶 3 (GSK-3)、叉头盒 O (FOXO) 和 ERK 的基础磷酸化(TCL1-551)。此外,R406 完全抑制 TCL1 白血病中抗 IgM 诱导的 Bcr 信号。尽管 TCL1 白血病中的组成型活性 Syk 水平较高,但 R406 对白血病细胞没有选择性细胞毒性。激酶测定:R406(R935788 的体外活性形式)在 DMSO 中连续稀释,然后在激酶缓冲液(20 mM HEPES,pH 7.4,5 mM MgCl2,2 mM MnCl2,1 mM DTT,0.1 mg/)中稀释至 1% DMSO mL 乙酰化 BGG)。在室温下添加激酶缓冲液中的 ATP 和底物,最终 DMSO 浓度为 0.2%。激酶反应在含有 5 μM HS1 肽底物和 4 μM ATP 的最终体积 20 μL 中进行,并通过在激酶缓冲液中添加 0.125 ng Syk 开始。使反应在室温下进行40分钟。通过添加 20 μL PTK 淬灭混合物来终止反应,该混合物含有用 FP 稀释缓冲液稀释的 EDTA/抗磷酸酪氨酸抗体(1× 最终)/荧光磷酸肽示踪剂(0.5× 最终)。将板在室温下黑暗中孵育 30 分钟,然后在 Polarion 荧光偏振板读数器上读数。使用通过与酪氨酸激酶测定试剂盒中提供的磷酸肽竞争剂竞争生成的校准曲线来转换数据以确定存在的磷酸肽的量。对于 IC50 测定,R406 在 11 个浓度下进行重复测试,并使用 Prism GraphPad 软件通过非线性回归分析进行曲线拟合。细胞测定:将细胞(TCL1-002、TCL1-252、TCL1-551、TCL1-870 和 TCL1-540)暴露于浓度不断增加的 R406(R935788 的体外活性形式)中 48 小时。通过碘化丙啶 (PI) 和膜联蛋白-A5-FITC 缀合物双重染色测定凋亡细胞的百分比。 Ki-67 染色使用 FITC 小鼠抗 Ki-67 套件进行。使用 CellQuest 3.3 版软件在 FACSCalibur 流式细胞仪上分析样品。
母药Fostamatinib Disodium无直接体外活性;所有体外活性均由代谢产物R406介导[1] - 抑制B细胞活化(R406介导):50 nM R406(来自Fostamatinib Disodium代谢)处理人B细胞72小时,抗IgM诱导的增殖减少85%;Western blot显示p-Syk(Tyr525/526)和p-LAT(Tyr191)分别降低90%/88%[2] - 阻断Fc受体(FcR)信号(R406介导):100 nM R406(来自200 nM Fostamatinib Disodium)处理人巨噬细胞24小时,IgG诱导的TNF-α分泌减少82%;FcR依赖的吞噬作用降低78%(流式细胞术,pHrodo标记微球)[2] - 抑制滑膜细胞炎症(R406介导):200 nM R406(来自300 nM Fostamatinib Disodium)处理人类风湿关节炎(RA)滑膜细胞2小时,IL-1β诱导的JNK磷酸化(Thr183/Tyr185)减少85%;qPCR检测显示MMP-1/MMP-3 mRNA表达降低75%/70%[3] |
| 体内研究 (In Vivo) |
在 Louvain 大鼠中,fostamatinib (R788) 具有高生物利用度且吸收迅速。单次口服R788 10 mg/kg或R406 20 mg/kg后,一小时后观察到以下结果:t1/2 = 4.2小时; AUC0-16 小时分别 = 10618 ngh/mL 和 30650 ngh/mL; Cmax 分别 = 2600 ng/mL 和 6500 ng/mL。血浆中不存在前药表明 R788 完全转化为 R406 [1]。
免疫性血小板减少症(ITP)小鼠模型([1]):8周龄雌性BALB/c小鼠腹腔注射抗血小板单克隆抗体(10 μg/只)诱导ITP。24小时后,小鼠口服灌胃Fostamatinib Disodium(30 mg/kg/天)持续14天;药物溶解于0.5%甲基纤维素+0.2%吐温80。每3天血细胞计数板检测血小板计数;HPLC定量血浆中R406浓度[1] - 小鼠被动皮肤过敏(PCA)模型([2]):8周龄雌性BALB/c小鼠耳皮内注射抗DNP IgE(1 μg/部位)。24小时后,小鼠口服灌胃Fostamatinib Disodium(50 mg/kg/天)持续7天;药物溶解于0.5%甲基纤维素。第8天,尾静脉注射DNP-BSA(1 mg/mL)激发过敏,1小时后数字卡尺测量耳肿胀[2] - 大鼠胶原诱导关节炎(CIA)模型([3]):6周龄雄性Lewis大鼠皮内注射牛II型胶原(100 μg/只,与完全弗氏佐剂乳化)诱导关节炎。诱导14天后,大鼠口服灌胃Fostamatinib Disodium(40 mg/kg/天)持续21天;药物溶解于0.5%甲基纤维素。每3天根据关节红肿、肿胀及活动度记录关节炎评分(0-10分)。实验结束后,踝关节4%多聚甲醛固定、切片,H&E染色进行组织病理学分析[3] |
| 酶活实验 |
Syk激酶活性实验(R406,[2]):重组人Syk激酶结构域(100 ng/孔)与R406(1-1000 nM,Fostamatinib Disodium的代谢产物)在反应缓冲液(25 mM HEPES pH 7.5,10 mM MgCl₂,1 mM DTT,0.1 mM 钒酸钠)中于37°C孵育30分钟。加入10 μM ATP和[γ-³²P]ATP,30°C继续孵育60分钟。反应产物点样于P81磷酸纤维素纸,0.75%磷酸洗涤3次,液体闪烁计数检测放射性。通过激酶活性抑制率的非线性回归计算IC50(41 nM)[2]
- 母药Fostamatinib Disodium无酶活实验数据(对Syk无活性)[1] |
| 细胞实验 |
人B细胞活化实验([2]):人外周血B细胞接种于96孔板(4×10³个/孔),Fostamatinib Disodium(50-500 nM,代谢为R406)预处理1小时后,抗IgM(10 μg/mL)刺激72小时。[³H]-胸腺嘧啶掺入法检测增殖;FITC标记抗CD69抗体流式细胞术分析CD69(活化标志物)表达[2]
- 人巨噬细胞FcR信号实验([2]):人外周血巨噬细胞接种于24孔板(1×10⁵个/孔),Fostamatinib Disodium(100-300 nM,转化为R406)预处理1小时后,IgG包被微球(微球:细胞=10:1)刺激24小时。收集上清液进行TNF-α ELISA检测;吞噬功能通过pHrodo标记IgG微球与细胞共孵育2小时后流式细胞术评估[2] - RA滑膜细胞炎症实验([3]):人RA滑膜细胞接种于6孔板(2×10⁵个/孔),Fostamatinib Disodium(200-500 nM,代谢为R406)预处理1小时后,IL-1β(10 ng/mL)刺激2小时。RIPA缓冲液裂解细胞,Western blot检测p-JNK(Thr183/Tyr185)。qPCR实验中,提取总RNA逆转录为cDNA,用特异性引物定量MMP-1/MMP-3 mRNA水平[3] |
| 动物实验 |
Dissolved in a 4 mg/mL solution in 0.1% carboxymethylcellulose sodium, 0.1% methylparaben, and 0.02% propylparaben (pH 6.5); 80 mg/kg; i.p. injection.
B6/C3H F1 female mice intraperitoneally injected with TCL1-002, TCL1-551, or TCL1-870 leukemia cells, and Eμ-TCL1 transgenic mice Mouse ITP model ([1]): 8-week-old female BALB/c mice were induced with anti-platelet monoclonal antibody (10 μg/mouse, i.p.). 24 hours later, mice were randomized to vehicle or Fostamatinib Disodium groups. Fostamatinib Disodium was administered via oral gavage at 30 mg/kg/day for 14 days; drug was dissolved in 0.5% methylcellulose + 0.2% Tween 80. Platelet counts were measured via hemocytometer every 3 days; plasma R406 concentrations were quantified via HPLC [1] - Mouse passive cutaneous anaphylaxis (PCA) model ([2]): 8-week-old female BALB/c mice were intradermally injected with anti-DNP IgE (1 μg/site) on the ears. 24 hours later, mice received Fostamatinib Disodium (50 mg/kg/day, oral gavage) for 7 days; drug was dissolved in 0.5% methylcellulose. On day 8, mice were challenged with DNP-BSA (1 mg/mL, i.v.), and ear swelling was measured via digital caliper 1 hour post-challenge [2] - Rat CIA model ([3]): 6-week-old male Lewis rats were intradermally injected with bovine type II collagen (100 μg/rat, emulsified in complete Freund’s adjuvant) to induce arthritis. 14 days post-induction, rats received Fostamatinib Disodium (40 mg/kg/day, oral gavage) for 21 days; drug was dissolved in 0.5% methylcellulose. Arthritis score (0-10, based on joint redness, swelling, and mobility) was recorded every 3 days. At study end, ankle joints were fixed in 4% paraformaldehyde, sectioned, and stained with H&E for histopathological analysis [3] |
| 药代性质 (ADME/PK) |
In humans ([1]): Oral bioavailability of Fostamatinib Disodium = 34% (100 mg dose); rapidly hydrolyzed to active R406 in the gastrointestinal tract and liver (t₁/₂ of parent drug = 1.2 hours, R406 = 3.5 hours). R406 reaches maximum plasma concentration (Cmax = 1.8 μM) at 2 hours post-oral administration of Fostamatinib Disodium [1]
- Distribution ([1]): R406 (metabolite) has a volume of distribution (Vd) = 11 L/kg; 97% bound to human plasma proteins (measured via ultrafiltration method) [1] - Excretion ([1]): 70% of R406 is excreted as inactive metabolites in feces, 25% in urine; no parent Fostamatinib Disodium is detected in excreta [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Human adverse events ([1]): Common treatment-related side effects include hypertension (18% of patients), diarrhea (15%), and nausea (10%); all are mild to moderate and manageable with dose adjustment (50-100 mg/day) [1]
- Hepatic safety ([1]): Mild, transient elevation of serum ALT/AST (<2× upper normal limit) occurs in 5% of patients [1] - Animal toxicity ([1][2]): In 28-day mouse studies, Fostamatinib Disodium (50 mg/kg/day, oral) caused no significant weight loss (>8%); serum BUN (17 ± 3 mg/dL) and creatinine (0.8 ± 0.1 mg/dL) remained within normal ranges [1] |
| 参考文献 | |
| 其他信息 |
Fostamatinib Disodium Anhydrous is the anhydrous form of fostamatinib disodium, an orally available Syk kinase inhibitor with potential anti-inflammatory and immunomodulating activities. Fostamatinib inhibits Syk kinase-mediated IgG Fc gamma receptor signaling, resulting in inhibition of the activation of mast cells, macrophages, and B-cells and related inflammatory responses and tissue damage. Syk kinase, widely expressed in hematopoietic cells, is a nonreceptor tyrosine kinase that is involved in coupling activated immunoreceptors to signal downstream events that mediate diverse cellular responses, including proliferation, differentiation, and phagocytosis.
Fostamatinib Disodium is an orally available disodium salt of the Syk kinase inhibitor fostamatinib with potential anti-inflammatory and immunomodulating activities. Fostamatinib inhibits Syk kinase-mediated IgG Fc gamma receptor signaling, resulting in inhibition of the activation of mast cells, macrophages, and B-cells and related inflammatory responses and tissue damage. Syk kinase, widely expressed in hematopoietic cells, is a nonreceptor tyrosine kinase that is involved in coupling activated immunoreceptors to signal downstream events that mediate diverse cellular responses, including proliferation, differentiation, and phagocytosis. See also: Fostamatinib (has active moiety). Drug Indication Tavlesse is indicated for the treatment of chronic immune thrombocytopenia (ITP) in adult patients who are refractory to other treatments. Fostamatinib Disodium (R788; Tavalisse) is an oral prodrug of R406, a selective spleen tyrosine kinase (Syk) inhibitor, approved by the FDA in 2018 for the treatment of adult immune thrombocytopenia (ITP) refractory to other therapies [1] - Mechanism of action: Fostamatinib Disodium is not pharmacologically active itself; it is rapidly converted to R406, which irreversibly binds to Syk, blocking B-cell receptor (BCR) signaling, Fc receptor-mediated immune responses, and proinflammatory cytokine secretion (e.g., TNF-α, IL-6) [1][2][3] - Preclinical data support efficacy in autoimmune diseases (rheumatoid arthritis, allergic inflammation) via R406-mediated Syk inhibition, but clinical approval is currently limited to ITP [1][2][3] |
| 分子式 |
C23H24FN6NA2O9P
|
|---|---|
| 分子量 |
624.42
|
| 精确质量 |
624.112
|
| CAS号 |
1025687-58-4
|
| 相关CAS号 |
Fostamatinib;901119-35-5;Fostamatinib disodium hexahydrate;914295-16-2
|
| PubChem CID |
25008120
|
| 外观&性状 |
White to yellow solid powder
|
| LogP |
3.53
|
| tPSA |
205.42
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
15
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
42
|
| 分子复杂度/Complexity |
893
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
HSYBQXDGYCYSGA-UHFFFAOYSA-L
|
| InChi Code |
InChI=1S/C23H26FN6O9P.2Na/c1-23(2)21(31)30(11-38-40(32,33)34)20-14(39-23)6-7-17(28-20)27-19-13(24)10-25-22(29-19)26-12-8-15(35-3)18(37-5)16(9-12)36-4;;/h6-10H,11H2,1-5H3,(H2,32,33,34)(H2,25,26,27,28,29);;/q;2*+1/p-2
|
| 化学名 |
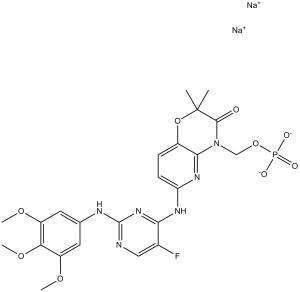
sodium (6-((5-fluoro-2-((3,4,5-trimethoxyphenyl)amino)pyrimidin-4-yl)amino)-2,2-dimethyl-3-oxo-2H-pyrido[3,2-b][1,4]oxazin-4(3H)-yl)methyl phosphate
|
| 别名 |
Fostamatinib disodium hexahydrate; R788; R 788; R-788 sodium; Tamatinib Fosdium, R-935788; R935788; R-935788; R 935788; R935788 sodium. Fostamatinib sodium, prodrug of R-406.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.00 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.00 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: 0.5% CMC+0.25% Tween 80,pH6.5:30 mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6015 mL | 8.0074 mL | 16.0149 mL | |
| 5 mM | 0.3203 mL | 1.6015 mL | 3.2030 mL | |
| 10 mM | 0.1601 mL | 0.8007 mL | 1.6015 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00798096 | Completed Has Results | Drug: Fostamatinib Disodium | T Cell Lymphoma | Rigel Pharmaceuticals | March 2009 | Phase 2 |
| NCT00923481 | Completed Has Results | Drug: Fostamatinib disodium | Head and Neck Neoplasms Pheochromocytoma |
National Cancer Institute (NCI) | April 2009 | Phase 2 |
| NCT02077192 | Completed Has Results | Drug: Fostamatinib Disodium | Immune Thrombocytopenic Purpura | Rigel Pharmaceuticals | October 2014 | Phase 3 |
| NCT00706342 | Completed Has Results | Drug: Fostamatinib Disodium / R935788 | Purpura, Thrombocytopenic, Idiopathic | Rigel Pharmaceuticals | January 2007 | Phase 2 |