| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
| 靶点 |
Estrogen Receptor/ER (IC50 = 9.4 nM)
Estrogen Receptor α (ERα): Fulvestrant (ICI 182780) binds to ERα with high affinity, exhibiting a Ki value of 0.2 nM in competitive ligand-binding assays; it does not bind to progesterone receptor (PR) or glucocorticoid receptor (GR) at concentrations up to 100 nM [1] - Estrogen Receptor β (ERβ): Fulvestrant inhibits ERβ-mediated transcriptional activity with an IC50 of 0.5 nM, and induces ERβ degradation in ERβ-positive breast cancer cells [3] |
|---|---|
| 体外研究 (In Vitro) |
FuLvestrant(ICI 182780;ZD 9238;ZM 182780)是一种非常有效的选择性雌激素作用抑制剂,在动物模型和人类乳腺癌细胞中显示出优异的生长抑制作用。 fuLvestrant 的 IC50 为 0.29 nM,可阻止 MCF-7 人乳腺癌细胞的发育。氟维司群的相对结合亲和力为 0.89。 FuLvestrant 保持其纯雌激素拮抗剂活性,同时具有显着增强的抗雌激素效力 [1]。氟维司群是一种下调 ER 的 ER 拮抗剂,是第一类新型内分泌控制药物[3]。 MCF-7 细胞中的 ERα 表达不受 1 μM ICI 47699 处理的影响,而被 100 nM FuLvestrant 完全抑制 [4]。
1. ER阳性乳腺癌细胞的抗增殖活性([1]): - 用氟维司群(0.1–100 nM)处理MCF-7(ERα阳性)乳腺癌细胞72小时,以浓度依赖方式抑制细胞增殖,细胞计数实验显示IC50为0.8 nM。10 nM浓度下,与雌激素处理组相比,集落形成率降低85%(集落形成实验)。它还可阻断雌激素诱导的PR表达(蛋白质印迹法:10 nM时降低90%)和ERE驱动的报告基因活性(荧光素酶实验:IC50=0.3 nM)[1] 2. 通过抑制自噬增强对他莫昔芬的敏感性([2]): - 在转染miR-214模拟物的ER阳性MDA-MB-231乳腺癌细胞中,用10 nM 氟维司群预处理24小时,可使他莫昔芬(1 μM)诱导的凋亡率提升40%(流式细胞术:Annexin V/PI染色)。蛋白质印迹法显示,与单独他莫昔芬组相比,氟维司群+miR-214使自噬相关蛋白LC3-II水平降低55%,Beclin-1降低60%,同时使切割型caspase-3水平提升70%,表明凋亡信号增强 [2] 3. ER降解活性([3]): - 在T47D(ERα阳性)乳腺癌细胞中,氟维司群(1–100 nM)以时间和浓度依赖方式诱导ERα降解。10 nM浓度下,24小时后ERα蛋白水平降低70%(蛋白质印迹法),48小时后降低90%。蛋白酶体抑制剂MG132(10 μM)可阻断该降解过程,表明ER的降解依赖蛋白酶体 [3] |
| 体内研究 (In Vivo) |
当单独给药时,氟维司群 (ICI 182,780) 在未成熟雌性大鼠中不表现出胃肠外 (sc) 促子宫活性。氟维司群在皮下注射剂量为 0.5 mg/kg/天时完全对抗雌激素的作用。口服氟维司群(5 mg/kg/天)治疗和皮下注射在质量上具有可比性[1]。在裸鼠的两个人类乳腺癌模型中。在其中一个模型中单次注射氟维司群 (5 mg) 后,MCF-7 肿瘤异种移植物的生长完全停止至少 4 周。在携带 MCF-7 异种移植物的裸鼠中进行的其他实验中,氟维司群抑制现有肿瘤生长的时间是 ICI 47699 治疗的两倍,并且延迟肿瘤生长的时间更长。大[3]。第 40 天时,氟维司群显示出 88% 的肿瘤生长抑制 (TGI) [4]。
1. MCF-7异种移植模型中的抗肿瘤疗效([1]): - 6–8周龄雌性裸鼠皮下接种5×10⁶ MCF-7细胞,当肿瘤体积达100 mm³时,以0.1、1、10 mg/只的剂量皮下注射氟维司群,每周1次,连续4周。10 mg/只剂量组较溶剂对照组抑制肿瘤生长80%(每周两次测量肿瘤体积)。肿瘤组织分析显示,ERα蛋白水平降低75%(蛋白质印迹法),增殖标志物Ki-67阳性率降低60%(免疫组化)[1] 2. ER阳性乳腺癌异种移植模型中的疗效([3]): - 携带T47D异种移植瘤(200 mm³)的去卵巢雌性裸鼠,每周1次皮下注射1 mg/只氟维司群,连续3周。与雌激素刺激对照组相比,肿瘤体积缩小50%。ELISA检测显示血清孕酮水平(雌激素活性标志物)降低45%,证实雌激素信号被抑制 [3] |
| 酶活实验 |
该实验室之前的研究描述了一系列具有纯抗雌激素活性的7α-烷基酰胺雌二醇类似物,以ICI 164384为例。现已鉴定出一种新化合物,7-α-[9-(4,4,5,5,5-五氟戊基亚磺酰基)壬基]雌-1,3,5(10)-三烯-3,17-β二醇(ICI 182780),其显著提高了抗雌激素效力并保留了纯雌激素拮抗剂活性。ICI 182780在未成熟大鼠中的抗子宫营养效力比ICI 164384高10倍以上(50%有效剂量分别为0.06和0.9mg/kg)。体内效力的这种数量级增加也部分反映在雌激素受体的内在活性上。与雌二醇(1.0)相比,ICI 182780和ICI 164384的相对结合亲和力分别为0.89和0.19。类似地,ICI 182780在MCF-7人乳腺癌症细胞中的体外生长抑制效力超过ICI 164384,其中分别记录了0.29和1.3nM的50%抑制浓度。ICI 182780是一种比4'-羟基三苯氧胺更有效的MCF-7生长抑制剂,在4'-羟三苯氧铵达到最大50%抑制的条件下,细胞数量减少了80%。与三苯氧胺处理的细胞相比,ICI 182780处理的细胞培养物中参与DNA合成的细胞比例显著降低,这反映了疗效的提高。[1]
由于其良好的耐受性,长期以来,内分泌疗法一直被认为是激素敏感性转移性癌症的首选治疗方法。然而,可用的选择性雌激素受体调节剂(如三苯氧胺)的雌激素激动剂作用,以及具有相似作用模式的内分泌疗法之间交叉耐药性的发展,导致需要通过不同机制发挥作用的新疗法。Fulvestrant(“Aslodex”)是一种新型内分泌治疗药物中的第一种,它是一种雌激素受体(ER)拮抗剂,可以下调ER,并且没有激动剂作用。本文概述了目前对ER信号传导的理解,并说明了氟维司群的独特作用方式。临床前和临床研究数据支持这种新型ER拮抗剂的新作用机制[3]。 ERα竞争配体结合实验([1]): 反应体系为200 μL,含50 ng/孔纯化重组人ERα、50 mM Tris-HCl缓冲液(pH 7.4)、10%甘油及0.5 nM [³H]-雌二醇(放射性配体)。加入浓度范围为0.01–100 nM的氟维司群,于4°C孵育18小时。通过 charcoal-dextran沉淀法(1%活性炭、0.1%葡聚糖,4°C孵育10分钟)去除未结合配体,随后离心(3000×g,10分钟)。采用液体闪烁计数器检测上清液放射性,通过Cheng-Prusoff方程计算Ki值 [1] |
| 细胞实验 |
细胞活力测定[2]
将接种在6孔板中的MCF7细胞用100 nM阴性对照或miR-214模拟物和抑制剂转染24小时。将细胞以8×103个细胞/孔的密度胰蛋白酶消化到96孔板中,然后用5μM的4-OHT或1μM的fulvestrant (FUL)处理72小时。通过3-[4,5-二甲基噻唑-2-基]-2,5-二苯基溴化四唑(MTT)法评估细胞存活率。 细胞自噬分析[2] 用GFP-LC3质粒(Addgene)转染细胞,然后用0.1%v/v乙醇载体或5μM 4-OHT或1μM氟维司群(FUL)处理48小时。在配备油浸透镜(40×)的共聚焦显微镜下,用405和488 nm激发激光观察GFP-LC3-II阳性斑点图案。使用Image J程序计数自噬体的数量 1. MCF-7细胞增殖与集落形成实验([1]): - 增殖实验:96孔板接种5×10³ MCF-7细胞,用含5%活性炭处理胎牛血清的无酚红RPMI 1640培养24小时后,加入氟维司群(0.1–100 nM)+1 nM雌二醇,孵育72小时。用血细胞计数板计数细胞,从剂量反应曲线计算IC50。 - 集落形成实验:6孔板接种1×10³ MCF-7细胞,加入氟维司群(0.1–10 nM)+1 nM雌二醇,孵育14天。甲醇固定集落,结晶紫染色后手动计数 [1] 2. MDA-MB-231细胞凋亡与自噬实验([2]): - 凋亡实验:24孔板接种2×10⁵ MDA-MB-231细胞,转染50 nM miR-214模拟物24小时后,加入氟维司群(10 nM)+他莫昔芬(1 μM)处理48小时。Annexin V-FITC/PI染色后,通过流式细胞术定量凋亡细胞(Annexin V+/PI-或Annexin V+/PI+)。 - 自噬实验:相同处理后提取总蛋白,蛋白质印迹法检测自噬标志物LC3-I/II和自噬相关蛋白Beclin-1,以β-肌动蛋白为内参 [2] 3. T47D细胞ERα降解实验([3]): - 6孔板接种3×10⁵ T47D细胞,用含5%活性炭处理胎牛血清的无酚红DMEM培养48小时后,加入氟维司群(1–100 nM)处理24或48小时。蛋白酶体抑制实验中,提前2小时加入10 μM MG132再给药。提取总蛋白,通过蛋白质印迹法(抗ERα一抗)检测ERα蛋白水平 [3] |
| 动物实验 |
Formulated to 50 mg/mL in arachis oil; 5 mg/mouse; s.c. injection
The human breast cancer xenografts MCF-7 in nude mice Sustained antiestrogenic effects, following a single parenteral dose of ICI 182,780 in oil suspension, were apparent in both rats and pigtail monkeys. In vivo, antitumor activity of ICI 182,780 was demonstrated with xenografts of MCF-7 and Br10 human breast cancers in nude mice. A single injection of ICI 182,780 provided antitumor efficacy equivalent to that of daily tamoxifen treatment for at least 4 weeks. The properties of ICI 182,780 identify this pure antiestrogen as a prime candidate with which to evaluate the potential therapeutic benefits of complete estrogen withdrawal in endocrine-responsive human breast cancer.[1] ICI 182,780/fulvestrant induced PKCε-dependent mechanical hyperalgesia[5] To substantiate the finding that the novel estrogen receptor GPR30 apparently mediates the recently described effect of estrogen on nociceptive neurons, we used a second agonist of GPR30, which simultaneously blocks signalling through ERα and -β, ICI 182,780 (DeFriend et al., 1994; Molinari et al., 2000; Chan et al., 2007). The behavioural experiments established a clear dose-dependence for intradermal ICI 182,780 to produce mechanical hyperalgesia (ICI 182,780 dissolved to 10 mg/mL in 100% DMSO, diluted to final concentration in 2.5 µL in PBS; final concentration of DMSO 10%) into hind paws of male rats. Neither spontaneous pain nor redness or swelling was observed. The maximum decrease in nociceptive threshold, by 35.3 ± 2.2%, was observed after injection of 100 ng ICI 182,780 (Fig. 6a; n = 6 paws; absolute value of baseline withdrawal threshold of negative controls 114 ± 1.6 g). 1. MCF-7 Xenograft Model ([1]): - Cell Inoculation: 5×10⁶ MCF-7 cells (suspended in 0.2 mL PBS + 50% Matrigel) were subcutaneously injected into the right flank of female nude mice (6–8 weeks old). - Drug Preparation: Fulvestrant was dissolved in sesame oil to concentrations of 0.1, 1, 10 mg/mL. - Administration: When tumors reached 100 mm³, mice were subcutaneously injected with Fulvestrant (0.1, 1, 10 mg/mouse) or sesame oil (control) once weekly for 4 weeks. - Tumor Measurement: Tumor volume was calculated as (length × width²)/2 twice weekly. After 4 weeks, mice were euthanized, and tumors were collected for Western blot and immunohistochemistry [1] 2. T47D Xenograft Model ([3]): - Ovariectomy: Female nude mice (6–8 weeks old) were ovariectomized 1 week before cell inoculation to eliminate endogenous estrogen. - Cell Inoculation: 2×10⁶ T47D cells (0.2 mL PBS + 50% Matrigel) were subcutaneously injected into the left flank. - Drug Administration: When tumors reached 200 mm³, mice were subcutaneously injected with Fulvestrant (1 mg/mouse, dissolved in sesame oil) once weekly for 3 weeks. A control group received 17β-estradiol (0.1 μg/mouse, subcutaneous) + sesame oil. - Sample Collection: After 3 weeks, mice were euthanized; tumors were weighed, and serum was collected for progesterone ELISA [3] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Fulvestrant was rapidly cleared by the hepatobiliary route with excretion primarily via the feces (approximately 90%). Renal elimination was negligible (less than 1%). 3 to 5 L/kg Peak plasma concentrations of fulvestrant are attained approximately 7 days after IM administration and persist for at least 1 month. Steady-state plasma fulvestrant concentrations usually are achieved within 3-6 months when the drug is administered once-monthly by IM injection. Fulvestrant appears to be rapidly and extensively distributed, principally into the extravascular space 99% (mainly VLDL, LDL, and HDL lipoprotein fractions). Has been shown to cross the placenta and distribute into milk in rats. For more Absorption, Distribution and Excretion (Complete) data for FULVESTRANT (8 total), please visit the HSDB record page. Metabolism / Metabolites Metabolism of fulvestrant appears to involve combinations of a number of possible biotransformation pathways analogous to those of endogenous steroids, including oxidation, aromatic hydroxylation, conjugation with glucuronic acid and/or sulphate at the 2, 3 and 17 positions of the steroid nucleus, and oxidation of the side chain sulphoxide. Identified metabolites are either less active or exhibit similar activity to fulvestrant in antiestrogen models. Studies using human liver preparations and recombinant human enzymes indicate that cytochrome P-450 3A4 (CYP 3A4) is the only P-450 isoenzyme involved in the oxidation of fulvestrant; however, the relative contribution of P-450 and non-P-450 routes in vivo is unknown. Biotransformation and disposition of fulvestrant in humans have been determined following intramuscular and intravenous administration of 14C-labeled fulvestrant. Metabolism of fulvestrant appears to involve combinations of a number of possible biotransformation pathways analogous to those of endogenous steroids, including oxidation, aromatic hydroxylation, conjugation with glucuronic acid and/or sulphate at the 2, 3 and 17 positions of the steroid nucleus, and oxidation of the side chain sulphoxide. Metabolites of fulvestrant exhibit pharmacologic activity that is similar to or less than that of the parent compound. In vitro studies indicate that CYP3A4 is the only enzyme involved in fulvestrant oxidation; however, the relative contribution of CYP and non-CYP routes in vivo currently is not known. Biological Half-Life 40 days The elimination half-life of fulvestrant is about 40 days. Oral Bioavailability: Fulvestrant has poor oral bioavailability (<5%) in mice and humans due to extensive first-pass metabolism; it is therefore administered via subcutaneous injection [3] - Plasma Half-Life: In nude mice, subcutaneous administration of Fulvestrant (1 mg/mouse) showed a plasma half-life of 48 hours, with peak plasma concentration (Cmax) of 80 ng/mL at 6 hours post-injection [3] - Tissue Distribution: Fulvestrant accumulates in ER-positive tumor tissues (tumor/plasma concentration ratio = 15:1) 24 hours after subcutaneous injection in xenograft models [3] |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Fulvestrant therapy is said to be associated with serum enzyme elevations in up to 15% of patients, but the elevations are generally asymptomatic, transient and mild, rarely requiring dose adjustment or discontinuation. ALT elevations above 5 times the upper limit of normal occurred in only 1% to 2% of patients. However, specifics on the timing and course of serum enzyme elevations during fulvestrant therapy have not been described. In addition, no cases of clinically apparent liver injury with jaundice were reported in the prelicensure controlled trials of fulvestrant and none have been published since its approval in the United States and more wide-scale use. Nevertheless, the product label for fulvestrant mentions that "hepatitis and liver failure have been reported infrequently ( Likelihood score: E (unproven but suspected cause of clinically apprent liver injury). Protein Binding 99% (mainly VLDL, LDL, and HDL) 1. In Vitro Cytotoxicity: - Fulvestrant (0.1–100 nM) showed no cytotoxicity in ER-negative MDA-MB-468 breast cancer cells (cell viability >95% vs. control, MTT assay) [1] - In ER-positive cells, its antiproliferative effect was specific to estrogen-stimulated growth, with no induction of non-specific cell death at therapeutic concentrations (1–10 nM) [3] 2. In Vivo Toxicity: - Subcutaneous administration of Fulvestrant (1–10 mg/mouse, 4 weeks) in nude mice did not cause significant changes in body weight, liver function (ALT, AST), or kidney function (BUN, creatinine) compared to control [1][3] - No signs of hematological toxicity (e.g., leukopenia, thrombocytopenia) were observed in treated mice [3] 3. Plasma Protein Binding: Fulvestrant has high plasma protein binding (>99%) in human and mouse plasma (measured via ultrafiltration) [3] |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Antineoplastic Agents; Hormonal Estrogen Antagonists Fulvestrant is indicated for the treatment of hormone receptor positive metastatic breast cancer in postmenopausal women with disease progression following antiestrogen therapy. /Included in US product label/ Drug Warnings /Fulvestrant is contraindicated in/ pregnancy, known hypersensitivity to fulvestrant, benzyl alcohol, or any ingredient in the formulation. Because fulvestrant is administered by IM injection, the drug should not be used in patients with bleeding diatheses or thrombocytopenia or in those receiving anticoagulant therapy. The most common adverse effects of fulvestrant are adverse GI effects (e.g., nausea, vomiting, constipation, diarrhea, abdominal pain), headache, back pain, vasodilation (hot flushes), and pharyngitis, which occurred in approximately 52, 15, 14, 18, and 16% of patients, respectively, who received the drug in clinical studies. Other adverse effects occurring in 5-23% of patients receiving fulvestrant (in order of descending frequency) include asthenia, pain, nutritional disorders, bone pain, dyspnea, injection site pain, increased cough, pelvic pain, anorexia, peripheral edema, rash, chest pain, flu syndrome, dizziness, insomnia, fever, paresthesia, urinary tract infection, depression, anxiety, and sweating. Injection site reactions with mild transient pain and inflammation were reported in 7% of patients receiving a single 5-mL injection of fulvestrant in one study and in 27% of those who received two 2.5-mL injections of the drug in another study. For more Drug Warnings (Complete) data for FULVESTRANT (7 total), please visit the HSDB record page. Pharmacodynamics Fulvestrant for intramuscular administration is an estrogen receptor antagonist without known agonist effects. 1. Drug Classification & Mechanism ([1][3]): - Fulvestrant is a pure estrogen receptor antagonist (SERM) with no agonist activity; it exerts effects by binding to ER, inducing receptor conformational changes, and promoting proteasome-dependent ER degradation (distinct from tamoxifen, which has partial agonist activity) [1][3] 2. Indications ([3]): - Approved for the treatment of hormone receptor-positive (HR+), advanced or metastatic breast cancer in postmenopausal women who have progressed on prior antiestrogen therapy (e.g., tamoxifen) [3] 3. Combination Efficacy ([2]): - miR-214 overexpression enhances the sensitivity of breast cancer cells to Fulvestrant by inhibiting autophagy (via downregulation of autophagy-related genes ATG5 and ATG7), suggesting a potential combination strategy for overcoming Fulvestrant resistance [2] |
| 分子式 |
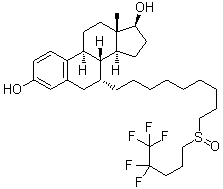
C32H47F5O3S
|
|
|---|---|---|
| 分子量 |
606.77
|
|
| 精确质量 |
606.316
|
|
| 元素分析 |
C, 63.34; H, 7.81; F, 15.66; O, 7.91; S, 5.28
|
|
| CAS号 |
129453-61-8
|
|
| 相关CAS号 |
Fulvestrant (Standard);129453-61-8;Fulvestrant (S enantiomer);1316849-17-8;Fulvestrant (R enantiomer);1807900-80-6;Fulvestrant-d3
|
|
| PubChem CID |
104741
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
674.8±55.0 °C at 760 mmHg
|
|
| 熔点 |
104-106°C
|
|
| 闪点 |
361.9±31.5 °C
|
|
| 蒸汽压 |
0.0±2.2 mmHg at 25°C
|
|
| 折射率 |
1.522
|
|
| LogP |
7.92
|
|
| tPSA |
76.74
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
9
|
|
| 可旋转键数目(RBC) |
14
|
|
| 重原子数目 |
41
|
|
| 分子复杂度/Complexity |
854
|
|
| 定义原子立体中心数目 |
6
|
|
| SMILES |
C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@@H](CC4=C3C=CC(=C4)O)CCCCCCCCCS(=O)CCCC(C(F)(F)F)(F)F
|
|
| InChi Key |
VWUXBMIQPBEWFH-WCCTWKNTSA-N
|
|
| InChi Code |
InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1
|
|
| 化学名 |
(7R,8R,9S,13S,14S,17S)-13-methyl-7-[9-(4,4,5,5,5-pentafluoropentylsulfinyl)nonyl]-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.75 mg/mL (4.53 mM) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.12 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浮液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: 2.08 mg/mL (3.43 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 配方 4 中的溶解度: ≥ 2.08 mg/mL (3.43 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100μL 20.8mg/mL澄清DMSO储备液加入900μL玉米油中,混合均匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 5 中的溶解度: 5% DMSO +95%Corn oil : 30mg/mL 配方 6 中的溶解度: 2.5 mg/mL (4.12 mM) in 15% Solutol HS 15 10% Cremophor EL 35% PEG 400 40% water (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6481 mL | 8.2404 mL | 16.4807 mL | |
| 5 mM | 0.3296 mL | 1.6481 mL | 3.2961 mL | |
| 10 mM | 0.1648 mL | 0.8240 mL | 1.6481 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。