| 规格 | 价格 | ||
|---|---|---|---|
| 500mg | |||
| 1g | |||
| Other Sizes |

| 靶点 |
Estrogen Receptor/ER
|
|---|---|
| 体外研究 (In Vitro) |
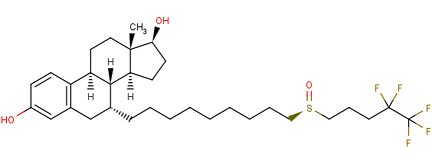
13-甲基-7-[9-(4,4,5,5,5-五氟戊基亚磺酰基)壬基]-6,7,8,9,11,12,14,15,16,17-十氢环戊[a]菲-3,17-二醇是一种类固醇。它具有雌激素的作用。
Fulvestrant(Faslodex®)由6-脱氢诺龙乙酸酯分四步合成(总收率35%)。在关键的C-C键形成步骤[1]中,使用了由市售9-溴壬-1-烯衍生的二茂锆的催化剂控制、室温、非对映选择性1,6-加成反应。 |
| 体内研究 (In Vivo) |
在大鼠和猪尾猴中,单次注射ICI 182780油悬浮液后,持续的抗雌激素作用都很明显。在体内,ICI 182780在裸鼠体内用MCF-7和Br10人乳腺癌异种移植物证明了其抗肿瘤活性。单次注射ICI 182780提供的抗肿瘤疗效相当于至少4周的每日他莫昔芬治疗。ICI 182780的特性将这种纯抗雌激素确定为主要候选药物,用于评估完全停止雌激素对内分泌反应性人类乳腺癌症的潜在治疗益处[2]。
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Fulvestrant was rapidly cleared by the hepatobiliary route with excretion primarily via the feces (approximately 90%). Renal elimination was negligible (less than 1%). 3 to 5 L/kg Peak plasma concentrations of fulvestrant are attained approximately 7 days after IM administration and persist for at least 1 month. Steady-state plasma fulvestrant concentrations usually are achieved within 3-6 months when the drug is administered once-monthly by IM injection. Fulvestrant appears to be rapidly and extensively distributed, principally into the extravascular space 99% (mainly VLDL, LDL, and HDL lipoprotein fractions). Has been shown to cross the placenta and distribute into milk in rats. For more Absorption, Distribution and Excretion (Complete) data for FULVESTRANT (8 total), please visit the HSDB record page. Metabolism / Metabolites Metabolism of fulvestrant appears to involve combinations of a number of possible biotransformation pathways analogous to those of endogenous steroids, including oxidation, aromatic hydroxylation, conjugation with glucuronic acid and/or sulphate at the 2, 3 and 17 positions of the steroid nucleus, and oxidation of the side chain sulphoxide. Identified metabolites are either less active or exhibit similar activity to fulvestrant in antiestrogen models. Studies using human liver preparations and recombinant human enzymes indicate that cytochrome P-450 3A4 (CYP 3A4) is the only P-450 isoenzyme involved in the oxidation of fulvestrant; however, the relative contribution of P-450 and non-P-450 routes in vivo is unknown. Biotransformation and disposition of fulvestrant in humans have been determined following intramuscular and intravenous administration of 14C-labeled fulvestrant. Metabolism of fulvestrant appears to involve combinations of a number of possible biotransformation pathways analogous to those of endogenous steroids, including oxidation, aromatic hydroxylation, conjugation with glucuronic acid and/or sulphate at the 2, 3 and 17 positions of the steroid nucleus, and oxidation of the side chain sulphoxide. Metabolites of fulvestrant exhibit pharmacologic activity that is similar to or less than that of the parent compound. In vitro studies indicate that CYP3A4 is the only enzyme involved in fulvestrant oxidation; however, the relative contribution of CYP and non-CYP routes in vivo currently is not known. Biological Half-Life 40 days The elimination half-life of fulvestrant is about 40 days. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Fulvestrant therapy is said to be associated with serum enzyme elevations in up to 15% of patients, but the elevations are generally asymptomatic, transient and mild, rarely requiring dose adjustment or discontinuation. ALT elevations above 5 times the upper limit of normal occurred in only 1% to 2% of patients. However, specifics on the timing and course of serum enzyme elevations during fulvestrant therapy have not been described. In addition, no cases of clinically apparent liver injury with jaundice were reported in the prelicensure controlled trials of fulvestrant and none have been published since its approval in the United States and more wide-scale use. Nevertheless, the product label for fulvestrant mentions that "hepatitis and liver failure have been reported infrequently ( Likelihood score: E* (unproven but suspected cause of clinically apprent liver injury). Protein Binding 99% (mainly VLDL, LDL, and HDL) |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Antineoplastic Agents; Hormonal Estrogen Antagonists Fulvestrant is indicated for the treatment of hormone receptor positive metastatic breast cancer in postmenopausal women with disease progression following antiestrogen therapy. /Included in US product label/ Drug Warnings /Fulvestrant is contraindicated in/ pregnancy, known hypersensitivity to fulvestrant, benzyl alcohol, or any ingredient in the formulation. Because fulvestrant is administered by IM injection, the drug should not be used in patients with bleeding diatheses or thrombocytopenia or in those receiving anticoagulant therapy. The most common adverse effects of fulvestrant are adverse GI effects (e.g., nausea, vomiting, constipation, diarrhea, abdominal pain), headache, back pain, vasodilation (hot flushes), and pharyngitis, which occurred in approximately 52, 15, 14, 18, and 16% of patients, respectively, who received the drug in clinical studies. Other adverse effects occurring in 5-23% of patients receiving fulvestrant (in order of descending frequency) include asthenia, pain, nutritional disorders, bone pain, dyspnea, injection site pain, increased cough, pelvic pain, anorexia, peripheral edema, rash, chest pain, flu syndrome, dizziness, insomnia, fever, paresthesia, urinary tract infection, depression, anxiety, and sweating. Injection site reactions with mild transient pain and inflammation were reported in 7% of patients receiving a single 5-mL injection of fulvestrant in one study and in 27% of those who received two 2.5-mL injections of the drug in another study. For more Drug Warnings (Complete) data for FULVESTRANT (7 total), please visit the HSDB record page. Pharmacodynamics Fulvestrant for intramuscular administration is an estrogen receptor antagonist without known agonist effects. Previous studies from this laboratory have described a series of 7 alpha-alkylamide analogues of estradiol with pure antiestrogenic activity, exemplified by ICI 164,384. A new compound, 7 alpha-[9-(4,4,5,5,5-pentafluoropentylsulfinyl)nonyl]estra-1,3,5(10 )- triene-3,17 beta-diol (ICI 182,780) has now been identified which has significantly increased antiestrogenic potency and retains pure estrogen antagonist activity. The antiuterotrophic potency of ICI 182,780 in the immature rat was more than 10-fold greater than that of ICI 164,384 (50% effective doses of 0.06 and 0.9 mg/kg, respectively). This order of magnitude increase of in vivo potency was also reflected, in part, by intrinsic activity at the estrogen receptor. The relative binding affinities of ICI 182,780 and ICI 164,384 were 0.89 and 0.19, respectively, compared with that of estradiol (1.0). Similarly, the in vitro growth-inhibitory potency of ICI 182,780 exceeded that of ICI 164,384 in MCF-7 human breast cancer cells, where 50% inhibitory concentrations of 0.29 and 1.3 nM, respectively, were recorded. ICI 182,780 was a more effective inhibitor of MCF-7 growth than 4'-hydroxytamoxifen, producing an 80% reduction of cell number under conditions where 4'-hydroxytamoxifen achieved a maximum of 50% inhibition. This increased efficacy was reflected by a greater reduction of the proportion of cells engaged in DNA synthesis in ICI 182,780-treated cell cultures compared with tamoxifen-treated cells. [2] |
| 分子式 |
C32H47F5O3S
|
|---|---|
| 分子量 |
606.77080655098
|
| 精确质量 |
606.316
|
| 元素分析 |
C, 63.34; H, 7.81; F, 15.66; O, 7.91; S, 5.28
|
| CAS号 |
1807900-80-6
|
| 相关CAS号 |
Fulvestrant;129453-61-8;Fulvestrant (S enantiomer);1316849-17-8
|
| PubChem CID |
104741
|
| 外观&性状 |
White powder ... the solution for injection is a clear, colorless to yellow, viscous liquid
|
| LogP |
9.2
|
| tPSA |
76.7
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
14
|
| 重原子数目 |
41
|
| 分子复杂度/Complexity |
854
|
| 定义原子立体中心数目 |
6
|
| SMILES |
S(CCCC(C(F)(F)F)(F)F)(CCCCCCCCCC1CC2C=C(C=CC=2C2CCC3(C)C(CCC3C21)O)O)=O
|
| InChi Key |
VWUXBMIQPBEWFH-WCCTWKNTSA-N
|
| InChi Code |
InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1
|
| 化学名 |
(7R,8R,9S,13S,14S,17S)-13-methyl-7-[9-(4,4,5,5,5-pentafluoropentylsulfinyl)nonyl]-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol
|
| 别名 |
13-methyl-7-[9-(4,4,5,5,5-pentafluoropentylsulfinyl)nonyl]-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol; Fulvestrant R enantiomer; 1807900-80-6; CID 3478439; (7R,8R,9S,13S,14S,17S)-13-methyl-7-[9-[(R)-4,4,5,5,5-pentafluoropentylsulfinyl]nonyl]-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol; 1316849-17-8; CID 138106603; SCHEMBL408338;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6481 mL | 8.2404 mL | 16.4807 mL | |
| 5 mM | 0.3296 mL | 1.6481 mL | 3.2961 mL | |
| 10 mM | 0.1648 mL | 0.8240 mL | 1.6481 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
A Global Study to Compare the Effects of Fulvestrant and Arimidex in a Subset of Patients With Breast Cancer.
CTID: NCT01602380
Phase: Phase 3 Status: Active, not recruiting
Date: 2024-11-27