| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
DPPH 的抗氧化活性通过葡萄糖胺(D-葡萄糖胺)表现出剂量依赖性[2]。 4 小时的葡萄糖胺处理可以降低翻译相关蛋白 p70S6K 和 S6 的磷酸化,并在蛋白水平上抑制 HIF-1α [3]。在阻塞的肾脏和用 TGF-β1 处理的肾细胞中,葡萄糖胺显着降低纤连蛋白、I 型胶原和 α-平滑肌肌动蛋白的肾表达 [4]。
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
In a pharmacokinetic study, glucosamine was 88.7% absorption by the gastrointestinal tract. Absolute oral bioavailability was 44%, likely due to the hepatic first-pass effect. In a pharmacokinetic study of 12 healthy adults receiving oral crystalline glucosamine, plasma levels increased up to 30 times the baseline levels and Cmax was 10 microM with a 1,500 mg once-daily dose. Tmax was about 3 hours. AUC was 20,216 ± 5021 after a 15,000 mg dose. Fecal excretion of glucosamine in a pharmacokinetic study was 11.3% within 120 hours after administration. Urinary elimination was found to be 1.19% within the first 8 hours post-administration. Results of a pharmacokinetic study of 12 healthy volunteers receiving three daily consecutive oral administrations of glucosamine sulfate soluble powder demonstrated glucosamine distribution to extravascular compartments. Human pharmacokinetic data for glucosamine is limited in the literature, however, a large animal model study of horses revealed a mean apparent volume of distribution of 15.4 L/kg. Concentrations of glucosamine ranged from 9-15 microM after an intravenous dose, and 0.3-0.7 microM after nasogastric dosing. These concentrations remained in the range of 0.1-0.7 microM in the majority of horses 12 hours after dosing, suggesting effectiveness of a once-daily dose. In rats and dogs, radioactivity from a C-14 labeled dose of glucosamine is detected in the liver, kidneys, articular cartilage, and other areas. Information on the absorption and serum pharmacokinetics for dietary glucosamine is very limited, and in some case, the available data are contradictory. For example, in one series of studies, (14)C-glucosamine was given orally to rats, dogs, and humans, and in all cases, the radiolabel was described as "efficiently" absorbed, reaching a plasma peak after about 4 hours. A high percentage of the radiolabel (about 35%) was excreted in the urine, and a similar amount was last in expired air. On the other hand, the laboratory that conducted this experiment was unable to detect chemical amounts of glucosamine in human serum after a single oral dose at 100 mg/kg (five times the clinical dose) using a chromatographic assay with a limit of detection of about 14 uM. This suggests that the bioavailable glucosamine in human serum after the normal recommended dosage (20 mg/kg) is well below 10 uM. About 90% of glucosamine administered orally as a glucosamine salt get absorbed from the small intestine, and from there it is transported via the portal circulation to the liver. It appears that a significant fraction of the ingested glucosamine is catabolized by first-pass metabolism in the liver. Free glucosamine is not detected in the serum after oral intake, and it si not presently known how much of an ingested dose is taken up in the joints in humans. Some uptake in the articular cartilage is seen in animal studies. Twelve healthy volunteers received three consecutive once-daily oral administrations of glucosamine sulfate soluble powder at the doses of 750, 1,500, and 3,000 mg, in an open, randomised, cross-over fashion. Glucosamine was determined in plasma collected up to 48 hr after the last dose. ... Endogenous plasma levels of glucosamine were detected (10.4-204 ng/ml, with low intra-subject variability). Glucosamine was rapidly absorbed after oral administration and its pharmacokinetics were linear in the dose range 750-1,500 mg, but not at 3,000 mg, where the plasma concentration-time profiles were less than expected based on dose-proportionality. Plasma levels increased over 30-folds from baseline and peaked at about 10 microM with the standard 1,500 mg once-daily dosage. Glucosamine distributed to extravascular compartments and its plasma concentrations were still above baseline up to the last collection time. Eighteen subjects with osteoarthritis were given 1,500 mg of commercial glucosamine sulphate after an overnight fast, and serum was then obtained at baseline and every 15-30 minutes over 3 hours, and additionally, from two subjects at 5 and 8 hours. Urine samples were collected at baseline and 3 hours after ingestion from three subjects. Baseline glucosamine was below the detection limit of 0.5 umol/L for all subjects, but after ingestion, glucosamine was detected in 17/18 subjects, beginning to rise at 30-45 minutes to a maximum at 90-180 minutes, with a range of 1.9-11.5 umol/L (0.34-2 ug/ml). For more Absorption, Distribution and Excretion (Complete) data for GLUCOSAMINE (7 total), please visit the HSDB record page. Metabolism / Metabolites Glucosamine undergoes metabolism in the liver. Metabolism information for glucosamine is limited in the literature. Biological Half-Life The estimated half-life for glucosamine is 15 hours after an oral dose. After a bolus intravenous injection of 1005 mg crystalline glucosamine sulfate, the parent drug has an apparent half life of 1.11 hours. Glucosamine elimination half-life /in twelve healthy volunteers/ was only tentatively estimated to average 15 hr. ... After i.v. administration the radioactivity due to glucosamine appears in plasma and is rapidly eliminated, with an initial half life of 0.28 hr. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In controlled trials, glucosamine and its combination with chondroitin have not been linked to serum enzyme elevations or to instances of clinically apparent liver injury. In addition, cases of clinically apparent liver injury have not been reported from prospective trials. Recently, several cases reports and small series of clinically apprent liver injury attributed to glucosamine (with or without chondroitin) have been published, but the relationship of glucosamine itself as opposed to other herbals in the implicated products or to potential contaminants, remains unclear and several cases were considered only "possibly" related to glucosamine. The time to onset is usually 1 to 4 weeks after starting the preparation and the pattern of injury is typically hepatocellular or mixed. At least one instance of acute liver failure has been reported. Immunoallergic features (rash, fever, eosinophilia) can occur, but are usually not prominent. Most patients were reported to recover within 4 to 8 weeks of stopping. There have not been instances of rechallenge with glucosamine, and the purity and concentration of glucosamine in the products used have not been reported. Likelihood score: D (possible rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Glucosamine is an amino-monosaccharide that is either derived from shellfish or synthetically produced. Glucosamine sulfate has no specific lactation-related uses. It is most commonly used to treat osteoarthritis. A glucosamine derivative, N-acetylglucosamine, is a normal component of human breastmilk. Glucosamine sulfate is well tolerated with occasional gastrointestinal discomfort (e.g., diarrhea, heartburn, nausea, vomiting) reported. Although no studies exist on the use of glucosamine sulfate during breastfeeding, its use by a nursing mother is unlikely to adversely affect the breastfed infant. Dietary supplements do not require extensive pre-marketing approval from the U.S. Food and Drug Administration. Manufacturers are responsible to ensure the safety, but do not need to prove the safety and effectiveness of dietary supplements before they are marketed. Dietary supplements may contain multiple ingredients, and differences are often found between labeled and actual ingredients or their amounts. A manufacturer may contract with an independent organization to verify the quality of a product or its ingredients, but that does not certify the safety or effectiveness of a product. Because of the above issues, clinical testing results on one product may not be applicable to other products. More detailed information about dietary supplements is available elsewhere on the LactMed Web site. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
| 参考文献 |
|
| 其他信息 |
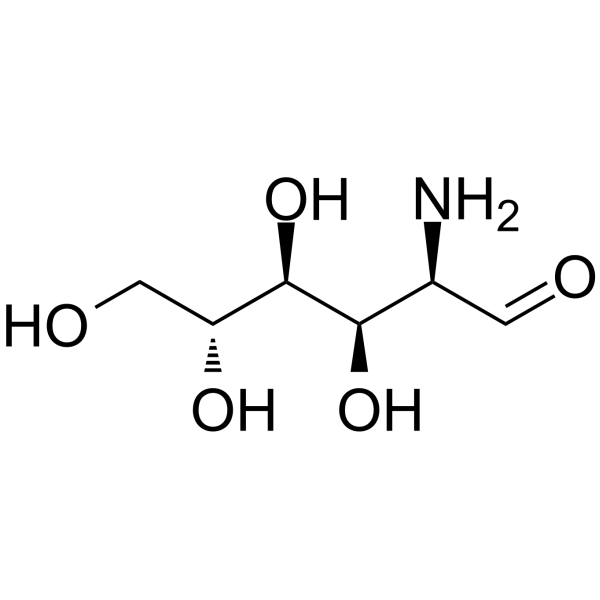
2-amino-2-deoxy-D-glucopyranose is a D-glucosamine whose structure comprises D-glucopyranose having an amino substituent at position 2. It has a role as an Escherichia coli metabolite, a mouse metabolite and a geroprotector. It is functionally related to a D-glucopyranose. It is a conjugate base of a 2-ammonio-2-deoxy-D-glucopyranose.
Osteoarthritis (OA) is a progressive and degenerative joint disease marked by loss of cartilage, bone changes, and synovial membrane inflammation. Treatment with chondroprotective drugs, such as glucosamine sulfate may offer additional benefits to nonsteroidal anti-inflammatory drugs treating the painful symptoms of OA. Glucosamine is commonly used over the counter as a treatment for arthritic joint pain, although its acceptance as a medical therapy varies due to contradictory and findings with unclear clinical significance during clinical trials. It is currently not approved as a prescription product by the FDA, but is widely available over the counter. Glucosamine is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). Glucosamine is a popular nutritional supplement and natural component of cartilage that is frequently combined with chondroitin sulfate and used for osteoarthritis and nonspecific joint pain. Glucosamine has been implicated in isolated case reports in causing clinically apparent liver injury, but the role of glucosamine as opposed to other herbal components or contaminants has not been shown, and liver injury due to glucosamine or chondroitin must be very rare if it occurs at all. D-Glucosamine has been reported in Daphnia pulex, Cannabis sativa, and other organisms with data available. See also: Glucosamine Hydrochloride (has salt form); Glucosamine Sulfate (has active ingredient); Poliglusam (monomer of) ... View More ... Drug Indication Glucosamine is generally used over the counter in the symptomatic treatment of osteoarthritis and joint pain, frequently combined with chondroitin sulfate and/or ibuprofen. Mechanism of Action The mechanism of action of glucosamine in joint health is unclear, however there are several possible mechanisms that contribute to its therapeutic effects. Because glucosamine is a precursor for glycosaminoglycans, and glycosaminoglycans are a major component of joint cartilage, glucosamine supplements may help to rebuild cartilage and treat the symptoms of arthritis. Some in vitro studies show evidence that glucosamine reduces inflammation via inhibition of interferon gamma and Nuclear factor kappa B subunit 65 (NF-κB p65), improving the symptoms of arthritis and joint pain. Clinical relevance is unknown at this time. When taken up by living cells, glucosamine reacts with ATP to form glucosamine-6-phosphate, the natural precursor of glycosaminoglycans (GAGs) that contain N-acetylglucosamine (keratan sulfate and Hyaluronan) and those that have N-acetylgalactosamine (heparan sulfate and chondroitin sulfate). These GAGs are polysaccharides composed of hexosamines and monosaccharides (e.g., galactose and glucuronic acid) arranged as a linear chain of repeating disaccharide units (such as the glucuronic acid and N-acetylgalactosamine-6-sulfate of chondroitin sulfate). With the exception of hyaluronan, GAGs do not exist alone in nature but are attached to specific "core" proteins, and the composite structures are called proteoglycans (protein-glycosaminoglycans). Both hyaluronan and many different kinds of proteoglycans (such as aggrecan, versican, and syndecan) are abundant throughout the body where they perform diverse functions. Therapeutic Uses Glucosamine and chondroitin sulfate are used to treat osteoarthritis. The multicenter, double-blind, placebo- and celecoxib-controlled Glucosamine/chondroitin Arthritis Intervention Trial (GAIT) evaluated their efficacy and safety as a treatment for knee pain from osteoarthritis. 1583 patients with symptomatic knee osteoarthritis /were randomly assigned/ to receive 1500 mg of glucosamine daily, 1200 mg of chondroitin sulfate daily, both glucosamine and chondroitin sulfate, 200 mg of celecoxib daily, or placebo for 24 weeks. Up to 4000 mg of acetaminophen daily was allowed as rescue analgesia. Assignment was stratified according to the severity of knee pain (mild [N=1229] vs. moderate to severe [N=354]). The primary outcome measure was a 20 percent decrease in knee pain from baseline to week 24. The mean age of the patients was 59 years, and 64 percent were women. Overall, glucosamine and chondroitin sulfate were not significantly better than placebo in reducing knee pain by 20 percent. As compared with the rate of response to placebo (60.1 percent), the rate of response to glucosamine was 3.9 percentage points higher (P=0.30), the rate of response to chondroitin sulfate was 5.3 percentage points higher (P=0.17), and the rate of response to combined treatment was 6.5 percentage points higher (P=0.09). The rate of response in the celecoxib control group was 10.0 percentage points higher than that in the placebo control group (P=0.008). For patients with moderate-to-severe pain at baseline, the rate of response was significantly higher with combined therapy than with placebo (79.2 percent vs. 54.3 percent, P=0.002). Adverse events were mild, infrequent, and evenly distributed among the groups. Glucosamine and chondroitin sulfate alone or in combination did not reduce pain effectively in the overall group of patients with osteoarthritis of the knee. Exploratory analyses suggest that the combination of glucosamine and chondroitin sulfate may be effective in the subgroup of patients with moderate-to-severe knee pain. Osteoarthritis (OA) is the most common form of arthritis, and it is often associated with significant disability and an impaired quality of life. To review all randomized controlled trials (RCTs) evaluating the effectiveness and toxicity of glucosamine in OA /the authors/ searched MEDLINE, PREMEDLINE, EMBASE, AMED, ACP Journal Club, DARE, CDSR, and the CCTR and also wrote letters to content experts, and hand searched reference lists of identified RCTs and pertinent review articles. All searches were updated in January 2005. Relevant studies met the following criteria: 1) RCTs evaluating the effectiveness and safety of glucosamine in OA, 2) Both placebo controlled and comparative studies were eligible, 3) Both single blinded and double blinded studies were eligible. Data abstraction was performed independently by two investigators and the results were compared for degree of agreement. Gotzsche's method and a validated tool were used to score the quality of the RCTs. Continuous outcome measures were pooled using standardized mean differences (SMD) as the measure of effect size. Dichotomous outcome measures were pooled using relative risk ratios (RR). MAIN RESULTS: Analysis restricted to eight studies with adequate allocation concealment failed to show benefit of glucosamine for pain and WOMAC function. Collectively, the 20 analyzed RCTs found glucosamine favored placebo with a 28% (change from baseline) improvement in pain (SMD -0.61, 95% CI -0.95, -0.28) and a 21% (change from baseline) improvement in function using the Lequesne index (SMD -0.51 95% CI -0.96, -0.05). However, the results are not uniformly positive, and the reasons for this remain unexplained. WOMAC pain, function and stiffness outcomes did not reach statistical significance.In the 10 RCTs in which the Rotta preparation of glucosamine was compared to placebo, glucosamine was found to be superior for pain (SMD -1.31, 95% CI -1.99, -0.64) and function using the Lequesne index (SMD -0.51, 95% CI -0.96, -0.05). Pooled results for pain (SMD -0.15, 95% CI -0.35, 0.05) and function using the WOMAC index (SMD 0.03, 95% CI -0.18, 0.25) in those RCTs in which a non-Rotta preparation of glucosamine was compared to placebo did not reach statistical significance. In the four RCTs in which the Rotta preparation of glucosamine was compared to an NSAID, glucosamine was superior in two, and equivalent in two. Two RCTs using the Rotta preparation showed that glucosamine was able to slow radiological progression of OA of the knee over a three year period (SMD 0.24, 95% CI 0.04, 0.43).Glucosamine was as safe as placebo in terms of the number of subjects reporting adverse reactions (RR=0.97, 95% CI, 0.88, 1.08). This update includes 20 studies with 2570 patients. Pooled results from studies using a non-Rotta preparation or adequate allocation concealment failed to show benefit in pain and WOMAC function while those studies evaluating the Rotta preparation show that glucosamine was superior to placebo in the treatment of pain and functional impairment resulting from symptomatic OA. WOMAC outcomes of pain, stiffness and function did not show a superiority of glucosamine over placebo for both Rotta and non-Rotta preparations of glucosamine. Glucosamine was as safe as placebo. Glucosamine may be indicated for the treatment and prevention of osteoarthritis, either by itself or in combination with chondroitin sulfate. Drug Warnings Those with type 2 diabetes and those who are overweight and have problems with glucose tolerance should have their blood sugars carefully monitored if they use glucosamine supplements. Because of insufficient safety data, children, pregnant women and nursing mothers should avoid using glucosamine. Side effects that have been reported are mainly mild gastrointestinal complaints such as heartburn, epigastric distress and diarrhea. No allergic reactions have been reported including sulfa-allergic reactions to glucosamine sulfate. /Glucosamine sulfate/ Glucosamine may increase insulin resistance and consequently affect glucose tolerance. Diabetics who ... decide to use glucosamine supplements will need to monitor their blood glucose and may need to adjust the doses of the medications they take to control blood glucose. ... The safety profile of glucosamine in the published studies ... is uniformly favorable and comparable to placebo. A few minor adverse events have been reported, including GI complaints such as heartburn, diarrhea, constipation, epigastric pain, and nausea. One concern about the use of glucosamine is its potential to cause or worsen diabetes. In animal models, increased glucosamine levels in cells have been associated with insulin resistance (a major factor in the genesis of Type 2 diabetes mellitus) and alterations in insulin production. Pharmacodynamics The administration of glucosamine, in theory, provides a building block towards the synthesis of glycosaminoglycans, slowing the progression of osteoarthritis and relieving symptoms of joint pain. Studies to this date examining the efficacy of glucosamine sulfate have been inconclusive. Glycosaminoglycans contribute to joint cartilage elasticity, strength, and flexibility. A systematic review of various studies and guidelines determined that modest improvements were reported for joint pain and function in patients taking glucosamine. A consistent joint space narrowing was observed, but with an unclear clinical significance. |
| 分子式 |
C6H13NO5
|
|---|---|
| 分子量 |
179.17
|
| 精确质量 |
179.079
|
| CAS号 |
3416-24-8
|
| 相关CAS号 |
Glucosamine sulfate;29031-19-4;Glucosamine hydrochloride;66-84-2
|
| PubChem CID |
439213
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 沸点 |
532.5±50.0 °C at 760 mmHg
|
| 熔点 |
88ºC
|
| 闪点 |
275.8±30.1 °C
|
| 蒸汽压 |
0.0±3.2 mmHg at 25°C
|
| 折射率 |
1.572
|
| LogP |
-2.38
|
| tPSA |
124.01
|
| 氢键供体(HBD)数目 |
5
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
12
|
| 分子复杂度/Complexity |
155
|
| 定义原子立体中心数目 |
4
|
| SMILES |
C([C@@H]1[C@H]([C@@H]([C@H](C(O1)O)N)O)O)O
|
| InChi Key |
MSWZFWKMSRAUBD-IVMDWMLBSA-N
|
| InChi Code |
InChI=1S/C6H13NO5/c7-3-5(10)4(9)2(1-8)12-6(3)11/h2-6,8-11H,1,7H2/t2-,3-,4-,5-,6?/m1/s1
|
| 化学名 |
(3R,4R,5S,6R)-3-amino-6-(hydroxymethyl)oxane-2,4,5-triol
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ~100 mg/mL (~558.13 mM)
DMSO : ~3.33 mg/mL (~18.59 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 50 mg/mL (279.06 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.5813 mL | 27.9065 mL | 55.8129 mL | |
| 5 mM | 1.1163 mL | 5.5813 mL | 11.1626 mL | |
| 10 mM | 0.5581 mL | 2.7906 mL | 5.5813 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。