| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
c-FMS (IC50 = 60 nM)
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:GW2580 在体外以 0.06 μM 完全抑制人 cFMS 激酶。 GW2580抑制CSF-1刺激的M-NFS-60骨髓肿瘤细胞、血清刺激的NSO骨髓肿瘤细胞、CSF-1刺激的新鲜分离的人单核细胞和VEGF刺激的人脐静脉血管内皮细胞的生长,IC50为0.33、13.5,分别为 0.47 和 12 μM。 1 μM GW2580 完全抑制 CSF-1 诱导的小鼠 M-NFS-60 骨髓细胞和人单核细胞的生长,并完全抑制人破骨细胞、大鼠颅骨和大鼠胎儿长骨培养物中的骨降解。 GW2580 抑制用 10 ng/mL 刺激的 RAW264.7 鼠巨噬细胞中的 CSF1R 磷酸化,IC50 约为 10 NM。 GW2580 还抑制 TRKA 活性,IC50 为 0.88 μM。激酶测定:将 10 μM 酶、100 μM ATP 和 5 mM MgCl2 在 50 mM Tris HCL 中室温孵育 90 分钟,通过自磷酸化激活酶。酶反应在 Biomek 2000 上使用圆底聚苯乙烯 96 孔板在 45 μL 体积中进行。将 1 μL DMSO 中的化合物或单独的 DMSO 添加到含有 30 μL 1.5× 底物反应混合物的每个孔中,其中含有50 mM Mops(3-[N-吗啡啉]丙磺酸),pH 7.5,15 mM MgCl2,6 μM 肽底物,生物素-EAIYAPFAKKK-NH2 7.5 mM DTT,75 mM NaCl,10 μM ATP 和 0.5 μCi(1 Ci = 37 GBq) [33P-γ] ATP 每次测定。通过添加 15 μL 稀释酶溶液启动反应,最终酶浓度为 20 nM。将 EDTA 添加到对照孔中以确定背景。反应进行40分钟,加入等体积的0.5%磷酸终止反应,将75 μL转移至已用100 μL 0.5%磷酸预润湿的96孔磷酸纤维素滤板中。将板在 Millipore 过滤板真空歧管上过滤,并用磷酸溶液洗涤 3 次,然后添加 40 μL 闪烁溶液。将板密封并在 Packard Topcount NXT 闪烁计数器中计数。细胞测定:在细胞生长测定开始前一天,将细胞离心并以每毫升 2×106 个细胞的浓度置于耗尽培养基中 24 小时。 M-NSF60 细胞的耗尽培养基缺乏 MCSF。第二天,将 10 mM DMSO 中的 GW2580 在含有 10% 血清的培养基中稀释至 20 μM 和 0.2% DMSO,并连续稀释以产生 10 点浓度曲线。将 M-NFS-60 细胞以 0.5×106 个细胞/mL 的浓度重悬于含有 10% 血清和 20 ng/mL 小鼠 MCSF 的培养基中。将细胞(50 μL)添加到含有抑制剂(50 μL)的每个孔中,3天后,将10 μL WST-1试剂添加到每个孔中。孵育 4 小时后,在 440 nm 处测量吸光度,并将生长计算为完全培养基的孔与耗尽培养基的孔之间的差异。
|
| 体内研究 (In Vivo) |
GW2580(在 CSF-1 引发剂量前 0.5 小时口服 40 mg/kg)可阻断外源性 CSF-1 将小鼠体内 LPS 诱导的 TNF-α 产生增加 63% 的能力。当在 CSF-1 引发之前给予小鼠时,GW2580 完全阻断 CSF-1 引发小鼠增加 IL-6 产生的能力。 GW2580 (80 mg/kg po) 完全抑制腹膜腔中 CSF-1 依赖性 M-NFS-60 肿瘤细胞的生长。在注射巯基乙酸盐前一周和注射巯基乙酸盐后的 4 天内,每天口服两次 GW2580 (80 mg/kg),可减少注射巯基乙酸盐后腹膜腔内巨噬细胞的积累(减少 45%)。在 21 天的佐剂关节炎模型中,GW2580(50 mg/kg)在第 0 至 21、7 至 21 或 14 至 21 天每天给药两次,可抑制关节结缔组织和骨质破坏。 Gw2580 (160 mg/kg) 通过抑制肿瘤从骨髓细胞中募集,诱导植入 3LL 肺肿瘤中总 CD45+ CD11b+ 骨髓细胞、CD11b+ F4/80+ TAM 和 CD11b+ Gr-1+ MDSC 减少 2 倍以上外周血。 GW2580 (80 mg/kg) 治疗能够抑制 Vegf-a(降低 35%)和 Mmp9(降低 70%)表达以及肿瘤血管密度(CD31 染色)。 GW2580 和抗 VEGFR-2 抗体的联合治疗可协同减少肿瘤生长。 DC101单独使用可减少35%的肿瘤生长,DC101和GW2580的组合可导致明显的协同肿瘤生长减少约70%。
|
| 酶活实验 |
室温下 90 分钟后,将 10 μM 酶、100 μM ATP 和 5 mM MgCl2 在 50 mM Tris HCL 中孵育,通过自磷酸化激活酶。 Biomek 2000 上的圆底聚苯乙烯 96 孔板用于进行 45 μL 酶反应。每孔中含有 30 mL 1.5 底物反应混合物,其中含有 50 mM Mops(3-[N-吗啉代]丙磺酸)、pH 7.5、15 mM MgCl2、6 M 肽底物和添加生物素-EAIYAPFAKKK-NH2,单独添加或在 1 mL DMSO 中添加。每次测定需要 0.5 μCi (1 Ci = 37 GBq) [33P-γ] ATP、75 mM NaCl、10 μM ATP 和 7.5 mM DTT。最终酶浓度为20 nM,加入15 μL稀释酶溶液开始反应。通过向对照孔中添加 EDTA 来确定背景。将 96 孔磷酸纤维素滤板用 100 μL 0.5% 磷酸预润湿,待反应进行 40 分钟并通过添加等体积的酸停止后,将 75 μL 反应液转移至其中。用磷酸溶液洗涤三轮并在 Millipore 过滤板真空歧管上过滤后,添加 40 μL 闪烁溶液。在 Packard Topcount NXT 闪烁计数器中,将板密封并计数。
|
| 细胞实验 |
在开始细胞生长测定之前,将细胞离心并用 2× 106 细胞/ml 稀释培养基注入 24 小时。 M-NSF60 细胞耗尽培养基不含 MCSF。第二天,将 10 mM 的 DMSO GW2580 连续稀释,以在含有 10% 血清的培养基中从 20 μM 和 0.2% DMSO 开始,产生 10 点浓度曲线。将 M-NFS-60 细胞重悬于培养基中后,将其与 10% 血清和 20 ng/mL 小鼠 MCSF 一起添加至 0.5×106 细胞/mL。每个孔中填充50μL含有抑制剂的细胞,然后,三天后,向每个孔中添加10μL WST-1试剂。 4 小时孵育期后,通过测量完全培养基的孔和耗尽培养基的孔之间的差异来确定生长情况。吸光度在 440 nm 处测量。
|
| 动物实验 |
Mouse myeloid carcinoma xenografts M-NFS-60
80 mg/kg Orally twice a day After oral administration, GW2580 blocked the ability of exogenous CSF-1 to increase LPS-induced IL-6 production in mice, inhibited the growth of CSF-1-dependent M-NFS-60 tumor cells in the peritoneal cavity, and diminished the accumulation of macrophages in the peritoneal cavity after thioglycolate injection. Unexpectedly, GW2580 inhibited LPS-induced TNF production in mice, in contrast to effects on monocytes and macrophages in vitro. In conclusion, GW2580's selective inhibition of monocyte growth and bone degradation is consistent with cFMS kinase inhibition. The ability of GW2580 to chronically inhibit CSF-1 signaling through cFMS kinase in normal and tumor cells in vivo makes GW2580 a useful tool in assessing the role of cFMS kinase in normal and disease processes.[1] In the present study, the kinase selectivity of GW2580 was further characterized, and the effects of chronic treatment were evaluated in normal and arthritic rats. GW2580 selectively inhibited cFMS kinase compared with 186 other kinases in vitro and completely inhibited CSF-1-induced growth of rat monocytes, with an IC(50) value of 0.2 microM. GW2580 dosed orally at 25 and 75 mg/kg 1 and 5 h before the injection of lipopolysaccharide inhibited tumor necrosis factor-alpha production by 60 to 85%, indicating a duration of action of at least 5 h. In a 21-day adjuvant arthritis model, GW2580 dosed twice a day (b.i.d.) from days 0 to 21, 7 to 21, or 14 to 21 inhibited joint connective tissue and bone destruction as assessed by radiology, histology and bone mineral content measurements. In contrast, GW2580 did not affect ankle swelling in the adjuvant model nor did it affect ankle swelling in a model where local arthritis is reactivated by peptidoglycan polysaccharide polymers. GW2580 administered to normal rats for 21 days showed no effects on tissue histology and only modest changes in serum clinical chemistry and blood hematology. In conclusion, GW2580 was effective in preserving joint integrity in the adjuvant arthritis model while showing minimal effects in normal rats.[3] |
| 参考文献 |
|
| 其他信息 |
Colony-stimulating-factor-1 (CSF-1) signaling through cFMS receptor kinase is increased in several diseases. To help investigate the role of cFMS kinase in disease, we identified GW2580, an orally bioavailable inhibitor of cFMS kinase. GW2580 completely inhibited human cFMS kinase in vitro at 0.06 microM and was inactive against 26 other kinases. GW2580 at 1 microM completely inhibited CSF-1-induced growth of mouse M-NFS-60 myeloid cells and human monocytes and completely inhibited bone degradation in cultures of human osteoclasts, rat calvaria, and rat fetal long bone. In contrast, GW2580 did not affect the growth of mouse NS0 lymphoblastoid cells, human endothelial cells, human fibroblasts, or five human tumor cell lines. GW2580 also did not affect lipopolysaccharide (LPS)-induced TNF, IL-6, and prostaglandin E2 production in freshly isolated human monocytes and mouse macrophages. After oral administration, GW2580 blocked the ability of exogenous CSF-1 to increase LPS-induced IL-6 production in mice, inhibited the growth of CSF-1-dependent M-NFS-60 tumor cells in the peritoneal cavity, and diminished the accumulation of macrophages in the peritoneal cavity after thioglycolate injection. Unexpectedly, GW2580 inhibited LPS-induced TNF production in mice, in contrast to effects on monocytes and macrophages in vitro. In conclusion, GW2580's selective inhibition of monocyte growth and bone degradation is consistent with cFMS kinase inhibition. The ability of GW2580 to chronically inhibit CSF-1 signaling through cFMS kinase in normal and tumor cells in vivo makes GW2580 a useful tool in assessing the role of cFMS kinase in normal and disease processes.[1]
Tumor-infiltrating myeloid cells (TIMs) support tumor growth by promoting angiogenesis and suppressing antitumor immune responses. CSF-1 receptor (CSF1R) signaling is important for the recruitment of CD11b(+)F4/80(+) tumor-associated macrophages (TAMs) and contributes to myeloid cell-mediated angiogenesis. However, the impact of the CSF1R signaling pathway on other TIM subsets, including CD11b(+)Gr-1(+) myeloid-derived suppressor cells (MDSCs), is unknown. Tumor-infiltrating MDSCs have also been shown to contribute to tumor angiogenesis and have recently been implicated in tumor resistance to antiangiogenic therapy, yet their precise involvement in these processes is not well understood. Here, we use the selective pharmacologic inhibitor of CSF1R signaling, GW2580, to demonstrate that CSF-1 regulates the tumor recruitment of CD11b(+)Gr-1(lo)Ly6C(hi) mononuclear MDSCs. Targeting these TIM subsets inhibits tumor angiogenesis associated with reduced expression of proangiogenic and immunosuppressive genes. Combination therapy using GW2580 with an anti-VEGFR-2 antibody synergistically suppresses tumor growth and severely impairs tumor angiogenesis along with reverting at least one TIM-mediated antiangiogenic compensatory mechanism involving MMP-9. These data highlight the importance of CSF1R signaling in the recruitment and function of distinct TIM subsets, including MDSCs, and validate the benefits of targeting CSF1R signaling in combination with antiangiogenic drugs for the treatment of solid cancers.[2] The cFMS (cellular homolog of the V-FMS oncogene product of the Susan McDonough strain of feline sarcoma virus) (Proc Natl Acad Sci U S A 83:3331-3335, 1986) kinase inhibitor 5-(3-methoxy-4-((4-methoxybenzyl)oxy)benzyl)pyrimidine-2,4-diamine (GW2580) inhibits colony-stimulating factor (CSF)-1-induced monocyte growth and bone degradation in vitro and inhibits CSF-1 signaling through cFMS kinase in 4-day models in mice (Proc Natl Acad Sci U S A 102:16078, 2005).[3] |
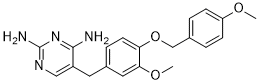
| 分子式 |
C20H22N4O3
|
|
|---|---|---|
| 分子量 |
366.41
|
|
| 精确质量 |
366.169
|
|
| 元素分析 |
C, 65.56; H, 6.05; N, 15.29; O, 13.10
|
|
| CAS号 |
870483-87-7
|
|
| 相关CAS号 |
|
|
| PubChem CID |
11617559
|
|
| 外观&性状 |
white solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
617.5±65.0 °C at 760 mmHg
|
|
| 闪点 |
327.2±34.3 °C
|
|
| 蒸汽压 |
0.0±1.8 mmHg at 25°C
|
|
| 折射率 |
1.635
|
|
| LogP |
2.66
|
|
| tPSA |
105.51
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
7
|
|
| 重原子数目 |
27
|
|
| 分子复杂度/Complexity |
433
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
N1C(N)=NC(N)=C(CC2C=C(OC)C(OCC3C=CC(OC)=CC=3)=CC=2)C=1
|
|
| InChi Key |
MYQAUKPBNJWPIE-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C20H22N4O3/c1-25-16-6-3-13(4-7-16)12-27-17-8-5-14(10-18(17)26-2)9-15-11-23-20(22)24-19(15)21/h3-8,10-11H,9,12H2,1-2H3,(H4,21,22,23,24)
|
|
| 化学名 |
5-[[3-methoxy-4-[(4-methoxyphenyl)methoxy]phenyl]methyl]pyrimidine-2,4-diamine
|
|
| 别名 |
GW 2580; SC-203877; SC 203877; GW 2580; GW-2580; 5-(3-Methoxy-4-((4-methoxybenzyl)oxy)benzyl)pyrimidine-2,4-diamine; 5-[[3-methoxy-4-[(4-methoxyphenyl)methoxy]phenyl]methyl]pyrimidine-2,4-diamine; GW632580X; GW 2580; SRV0JCF9LI; SC203877
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (5.68 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (5.68 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (5.68 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 5% DMSO+30% PEG 300+5% Tween 80+ddH2O: 5 mg/mL 配方 5 中的溶解度: 5 mg/mL (13.65 mM) in 0.5% CMC-Na/saline water (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.7292 mL | 13.6459 mL | 27.2918 mL | |
| 5 mM | 0.5458 mL | 2.7292 mL | 5.4584 mL | |
| 10 mM | 0.2729 mL | 1.3646 mL | 2.7292 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Angiogenesis and growth kinetics of tumors treated with GW2580. Blood. 2010 Feb 18; 115(7): 1461–1471. |
TIMs mediate MMP-9 induction by anti–VEGFR-2 therapy. Blood. 2010 Feb 18; 115(7): 1461–1471. |
Targeting MDSC infiltration and tumor angiogenesis in orthotopic RM-1 prostate tumors. Blood. 2010 Feb 18;115(7):1461-71. |