| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
研究了不同剂量(300 和 500 mg/mL)的乙醇酸 (GA) 或 LA 对人和小鼠黑色素瘤细胞发育的影响。即使每种药物浓度为 300 mg/mL,五天后,两种类型的细胞仍能有效扩增。另一方面,500 mg/mL LA 和 500 mg/mL 乙醇酸均能减少人和小鼠黑色素瘤细胞的发育(分别为 27% 和 36%)[1]。
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The penetration of 10% aq. glycolic acid, adjusted to pH 3.8 using either ammonium or sodium hydroxide, was examined using separated Yucatan minipig epidermis and full thickness hairless mouse skin. A 200 uL-aliquot of each formulation was applied to an area of a Franz diffusion cell, and glycolic acid was analyzed using liquid scintillation counting. Using an occlusive patch, penetration was linear with a lag time of less than 15 mm. After 8 hr, 0.8 and 1.6% of the ammonium and sodium salts penetrated, respectively, using the pig skin model and 1.8 and 2.3% of the ammonium and sodium salts penetrated, respectively, using the mouse skin model. Under open patch conditions, penetration was not linear and lag time was greater than 15 mm. Using the pig skin model, 1.1 and 0.7% of the ammonium and sodium salts penetrated, respectively, and using the mouse skin model, 0.6 and 0.9% of the ammonium and sodium salts penetrated, respectively. The skin penetration of (14)C-glycolic acid was studied using an in vitro system in which a cream formulation was applied to pig skin at a dose of 5 mg/0.79 sq cm skin without an occlusive patch. It was determined that 3.1% of the applied glycolic acid penetrated the skin. Two female rhesus monkeys were dosed orally with 4 mL/kg of 500 mg/kg homogenous 1-(14)C-glycolic acid, 0.73 uC/mmol, in aq. solution via stomach tube. Urine was collected at intervals of 0-8, 8-24, 24-48, 48-72, and, for one monkey, 72-96 hr. Over a 72 hr period one animal excreted, as a percentage of the dose, 53.2% (14)C, 51.4% of which was excreted in the urine; 51.4% of the dose was excreted in the first 24 hr. The second animal excreted a total of 42.2% (14)C over 96 hr, 36.6% of which was excreted in the urine; 34.1% of the dose was excreted in the first 24 hr. (The greater amount of fecal radioactivity observed for this monkey could have been due to urinary radioactivity contamination.) Very little of the dose was converted to radioactive glyoxylic, hippuric, or oxalic acid. Skin penetration of 10% aq. Glycolic acid was determined in vitro using human female (age 87 years) abdominal skin. The aq. solution was prepared by adding 0.8 mL 12.473% glycolic acid solution to 0.2 mL of (2-(14)C) glycolic acid solution, 44 mCi/mmol or 250 iCi/mL that contained 0.216 mg glycolic acid. The pH of a mixture containing 0.8 mL of the 12.473% glycolic acid solution and 0.2 mL of water was 3.72. Skin integrity was assessed by determining the permeability coefficient of tritiated water. Twenty uL of 10% aq. glycolic acid solution, 2 mg active, was placed on the stratum corneum surface; 13 replicates were used. Samples of 200 uL, which were taken 1, 2, 4, 6, 8, and 24 hr after application, were counted using a liquid scintillation counter. The skin surface was rinsed 3 times after the 24 hr sample was taken. The average total absorption over 24 hr 2.6 +/= 0.37 ug/sq cm representing 0.15 +/= 0.02% of the applied dose. A lag time of approximately 3.8 hr was followed by a period of steady-state diffusion at a rate of 0.13 ug/sq cm/hr. After 24 hr, 48 +/= 0.05% of the dose was recovered in the skin and 0.15 +/= 0.02% was found in the receptor phase. Total recovery was 102.9% +/= 2.9%. For more Absorption, Distribution and Excretion (Complete) data for HYDROXYACETIC ACID (14 total), please visit the HSDB record page. Metabolism / Metabolites The kinetics of orally administered ethylene glycol (EG) and its major metabolites, glycolic acid (GA) and oxalic acid (OX), in pregnant (P; gestation day 10 at dosing, GD 10) rats were compared across doses, and between pregnant and nonpregnant (NP) rats. Groups of 4 jugular vein-cannulated female rats were administered 10 (P and NP), 150 (P), 500 (P), 1000 (P), or 2500 (P and NP) mg (13)C-labelled EG/kg body weight. Serial blood samples and urine were collected over 24-hr postdosing, and analyzed for EG, GA, and OX using GC/MS techniques. Pharmacokinetic parameters including Cmax, Tmax, AUC, and beta-t(1/2) were determined for EG and GA. Pregnancy status (GD 10-11) had no impact on the pharmacokinetic parameters investigated. Blood levels of GA were roughly dose-proportional from 10 to 150 mg EG/kg, but increased disproportionately from 500 to 1000 mg EG/kg. EG and GA exhibited dose-dependent urinary elimination at doses > or = 500 mg EG/kg, probably due to saturation of metabolic conversion of EG to GA, and of GA to downstream metabolites. The shift to nonlinear kinetics encompassed the NOEL (500 mg EG/kg) and LOEL (1000 mg EG/kg) for developmental toxicity of EG in rats, providing additional evidence for the role of GA in EG developmental toxicity. The peak maternal blood concentration of GA associated with the LOEL for developmental toxicity in the rat was quite high (363 microg/g or 4.8 mM blood). OX was a very minor metabolite in both blood and urine at all dose levels, suggesting that OX is not important for EG developmental toxicity. The disposition of dichloroacetic acid (DCA) was investigated in Fischer 344 rats over the 48 hr after oral gavage of 282 mg/kg of 1- or 2-(14C)DCA (1-DCA or 2-DCA) and 28.2 mg/kg of 2-DCA... The major urinary metabolites were glycolic acid, glyoxylic acid, and oxalic acid. DCA and its metabolites accumulated in the tissues and were eliminated slowly.... The accumulation of glycolate and the elimination kinetics of ethylene glycol (EG) /was examined in/ ... male Sprague-Dawley rats and mixed breed dogs... . EG was administered by gavage ... . The peak plasma level of EG occurred at 2 hr after dosing and that of glycolate between 4-6 hr. The rate of EG elimination was somewhat faster in rats with a half-life of 1.7 hr compared to 3.4 hr in dogs. The maximum plasma level of glycolate was greater in rats, although the pattern of accumulation was similar to that in dogs. Glycolate disappeared from the plasma at the same time as EG, suggesting a slower rate of elimination of the metabolite than that of EG. Renal excretion of EG was an important route for its elimination, accounting for 20-30% of the dose. Renal excretion of glycolate represented about 5% of the dose... /Glycolate/ 1,2-(14)C-Ethylene glycol (EG) was given to female CD (Sprague-Dawley) rats and CD-1 mice in order to determine tissue distribution and metabolic fate after intravenous (iv), peroral (po), and percutaneous (pc) doses. Rats were given doses of 10 or 1000 mg/kg by each route, and additional pc doses of 400, 600 or 800 mg/kg. Mice were also given iv and po doses of 10 or 1000 mg/kg, and intermediate po doses of 100, 200 or 400 mg/kg. Mice were given po doses of 100 or 1000 mg/kg, and both species were given a 50% (w/w) aqueous po dose to simulate antifreeze exposure. For both species, EG is very rapidly and almost completely adsorbed after po doses. ... The tissue distribution of EG following either iv or po routes was essentially the same, with similar percentages recovered for each dose by both routes and for either species. Cutaneously-applied EG was slowly and rather poorly adsorbed in both species, in comparison with po-dose administration, and urinalysis after undiluted po doses indicated that EG probably penetrates rat skin in the parent form. There was an absence in both species of dose-dependent changes in disposition and elimination following the pc application of EG. (14)C-labelled EG, glycolic acid and/or oxalic acid accounted for the majority of the detectable radioactivity in the urine samples from all dose routes in the rat, while glycoaldehyde and glyoxylic acid were not detected in any of the urine fractions evaluated. Similar increases in glycolate production with increasing dose were also observed in mouse urine samples from iv and po dosing. Also, glyoxylate and oxalate were absent from mouse urine... For more Metabolism/Metabolites (Complete) data for HYDROXYACETIC ACID (9 total), please visit the HSDB record page. The main path of the degradation of glycolic acid is to glyoxylic acid. This reaction is mediated by lactic dehydrogenase or glycolic acid oxidase. Once glyoxylic acid is formed, it is apparently degraded very rapidly to a variety of products, a few of which have been observed. Its breakdown to 2-hydroxy-3-oxoadipate it is thought, is mediated by thiamine pyrophosphate in the presence of magnesium ions. The formation of glycine involves pyridoxal phosphate and glyoxylate transaminase, whereas the formation of carbon dioxide and water via formic acid apparently involves coenzyme A (CoA) and flavin mononucleotides. (T29) Biological Half-Life ... ethylene glycol and glycolate were distributed in total body water with plasma half-lives of 8.4 and 7.0 hr respectively. Rats given 1, 5, and 10 mL/kg diethylene glycol eliminated diethylene glycol in their urine with half lives of 6, 6, and 12 hr assuming first order kinetics. More detailed analysis showed that 6, 9, and 18 hr after dosing with 1, 5, and 10 mL/kg diethylene glycol elimination of (14)C activity followed zero order kinetics then changed to first order kinetics with a half life of 3 hr. Rats dosed with 3 and 5 mL/kg ethylene glycol excreted unchanged ethylene glycol in their urine with half lives of 4.5 and 4.1 hr respectively. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Hydroxyacetic (glycolic) acid is an odorless, colorless and translucent solid. The primary uses of hydroxyacetic acid are in cleaning and metal processing. Other specialized applications include biomedical uses, printed wire board flux, adhesives, textiles, hydrogen sulfide abatement, tanning, oil well acidification, and biodegradable polymers and copolymers for absorbable sutures and drug delivery systems. It is also used in skin care products as exfolliant and keratolytic. HUMAN EXPOSURE AND TOXICITY: Inhalation may cause irritation of mucous membranes with upper respiratory and bronchial irritation. Skin contact may cause severe skin irritation with discomfort or rash. Higher or prolonged exposure may cause skin burns or ulceration. Eye contact may cause eye corrosion with corneal or conjunctival ulceration. Permanent eye damage can occur. Ingestion may cause corrosion of mucous membranes with stomach discomfort, nausea, and prostration. Kidney damage or fatality may occur from gross overexposure. ANIMAL TOXICITY STUDIES: A basal diet with 3% glycolic acid for 3 weeks in rats resulted in a high incidence of oxalate urolithiasis (mostly in the kidneys, but some animals also had uroliths in the ureter and urinary bladder. Also, fine crystalline depositions were present throughout the cortex and medulla and clusters of concretions were on the surface or embedded in the renal papilla. In dogs given daily oral doses of 1000 mg glycolic acid for 35 days, no abnormal secretions of oxalic acid were found and no damage to the gastroenteric tract or kidneys was reported. In other experiment, rats were administered up to 600 mg/kg/day of the test substance by gavage for 90 days. Two deaths occurred in males at 600 mg/kg/day. Decreased mean body weight, overall body weight gain, food consumption, and food efficiency occurred in males and females of the 300 and 600 mg/kg/day groups. Microscopic findings of oxalate crystal nephrosis and unilateral hydronephrosis, and hyperplasia of the transitional epithelium of the renal pelvis were also observed (in males only) at these dose levels. No organ weight, gross or microscopic findings indicative of systemic toxicity were observed in female rats exposed to 300 or 600 mg/kg/day. The developmental toxicity of glycolic acid was assessed in rats over days 7-21 of gestation. Groups of mated female rats were gavaged at daily dose levels of up to 600 mg/kg. Clear evidence of maternal toxicity was demonstrated at 600 mg/kg. There was marked evidence of developmental toxicity at 600 mg/kg. Mean fetal weight was statistically significantly reduced while the incidences of skeletal (ribs, vertebra, and sternebra) malformations and variations were statistically significantly increased. Glycolic acid was not found to be genotoxic based on negative Ames test with and without activation using Salmonella typhimurium TA98, TA100, TA1535, TA1537, and TA1538. ECOTOXICITY STUDIES: Green Algae were exposed to glycolic acid for 72 hours. At the end of the exposure period, a control replicate and samples from the test concentrations exhibiting a 50% or greater inhibition of cell counts were selected for a recovery test and exposed to nutrient medium for an additional 144 hours. The effects upon growth rate and biomass were found to be algistatic. Fathead minnows were exposed to glycolic acid for 96 hours under static conditions. All deaths occurred within 24 hours. Daphnia magna were exposed to glycolic acid for 48 hours under static conditions. There were no sublethal effects observed in the surviving daphnids. Glycolic acid's toxicity is due to its metabolism to oxalic acid. Glycolic and oxalic acid, along with excess lactic acid, are responsible for the anion gap metabolic acidosis. Oxalic acid readily precipitates with calcium to form insoluble calcium oxalate crystals. Tissue injury is caused by widespread deposition of oxalate crystals and the toxic effects of glycolic acid. (A612, A613) Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of glycolic acid (hydroxyacetic acid) on the skin during breastfeeding. Because it is unlikely to be appreciably absorbed or appear in breastmilk, it is considered safe to use during breastfeeding. Avoid application to areas of the body that might come in direct contact with the infant's skin or where the drug might be ingested by the infant via licking. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Toxicity Data LC50 (rat) = 7.1 mg/m3/4hr LD50: 1950 mg/kg (Oral, Rat) (A655) LD50: 1000 mg/kg (Intravenous, Cat) (A730) LC50: 7.7-14 mg/L over 4 hours (Inhalation, Rat) (A730) Interactions The effect of 0.35 to 0.8 mmol/kg glycolic acid and 1.0 to 4.4 mmol/kg sodium glycolate on cyclopropane-epinephrine induced cardiac arrhythmias was examined using dogs. Doses of 0.35 to 0.5 mmol/kg glycolic acid increased the duration of arrhythmias in the 13 dogs tested, whereas doses >0.5 mmol/kg decreased or totally eliminated the arrhythmias in each of 11 dogs. Depression was observed for many of the dogs at higher doses. Sodium glycolate was much less effective in decreasing the arrhythmias, with 3 mmol/kg being required and its action being transient. ... This study was performed in order to determine whether short-term dermal treatment with glycolic acid, a representative alpha-hydroxy acid (AHA), can enhance the damaging effects of UV light. The duration of the effect of AHAs on the sensitivity of skin to UV light was also examined. ... The backs of 29 Caucasian subjects were treated, once daily, 6 days per week with either 10% glycolic acid (pH 3.5) or placebo in a randomized double-blinded study. At the end of 4 weeks, sites within each treated area were exposed to 1.5 MED of UV light, determined on previously untreated skin. Specimens were obtained for enumeration of sunburn cells (SBCs) in the first group of subjects (n = 16), whereas cyclobutyl pyrimidine dimers (CPDs) in DNA were determined in the second group (n = 13). The minimal erythema dose (MED) in each site was also determined in the first group of subjects. Sunburn cells and MEDs were re-evaluated in the first group 1 week after discontinuing AHA applications. ... Glycolic acid caused enhanced sensitivity to UV light measured as increased SBC induction and lowered MEDs. Cyclobutyl pyrimidine dimers were elevated but not to a statistically significant level. No differences in SBCs or MEDs were evident after a week of discontinued treatments... Hairless mice were irradiated thrice weekly for 10 weeks with UVB. In the 10-week postirradiation period, the mice were treated topically five times per week with tretinoin (0.05%), glycolic acid (10%), benzalkonium chloride (1.0%), sodium lauryl sulfate (5%), croton oil (5%) and the water - propylene glycol vehicle... Tretinoin-treated skin had increased amounts of collagen and type III procollagen whereas irritant- and peeling agent-treated skins were similar to vehicle-treated controls. Glycolic acid, a depressant antagonizing the convulsant action of strychnine in spinal cord of cats. For more Interactions (Complete) data for HYDROXYACETIC ACID (11 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat oral 4240 mg/kg bw LD50 Rat oral 1,600-3200 mg/kg bw LD50 Rat oral 1,950 mg/kg LD50 Guinea pig oral 1,920 mg/kg For more Non-Human Toxicity Values (Complete) data for HYDROXYACETIC ACID (17 total), please visit the HSDB record page. |
| 参考文献 |
[1]. Usuki A, et al. The inhibitory effect of glycolic acid and lactic acid on melanin synthesis in melanoma cells. Exp Dermatol. 2003;12 Suppl 2:43-50
|
| 其他信息 |
Glycolic acid is a 2-hydroxy monocarboxylic acid that is acetic acid where the methyl group has been hydroxylated. It has a role as a metabolite and a keratolytic drug. It is a 2-hydroxy monocarboxylic acid and a primary alcohol. It is functionally related to an acetic acid. It is a conjugate acid of a glycolate.
Glycolic acid is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). Glycolate is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). Glycolic acid has been reported in Populus tremula, Psychotria punctata, and other organisms with data available. Glycolic acid (or hydroxyacetic acid) is the smallest alpha-hydroxy acid (AHA). This colorless, odorless, and hygroscopic crystalline solid is highly soluble in water. Due to its excellent capability to penetrate skin, glycolic acid finds applications in skin care products, most often as a chemical peel. It may reduce wrinkles, acne scarring, hyperpigmentation and improve many other skin conditions, including actinic keratosis, hyperkeratosis, and seborrheic keratosis. Once applied, glycolic acid reacts with the upper layer of the epidermis, weakening the binding properties of the lipids that hold the dead skin cells together. This allows the outer skin to dissolve revealing the underlying skin. (L1909) See also: Glycolic acid; salicylic acid; sulfur (component of); Glycolic acid; salicylic acid (component of); Glycerin; glycolic acid (component of) ... View More ... Mechanism of Action Ethylene glycol toxicity results from its metabolism to glycolic acid and other toxic metabolites. The accumulation of glycolate and the elimination kinetics of ethylene glycol and its metabolites are not well understood, so studies with male Sprague-Dawley rats and mixed breed dogs have been carried out. Ethylene glycol was administered by gavage to rats and dogs which were placed in metabolic cages for urine and blood sample collection at timed intervals. The peak plasma level of ethylene glycol occurred at 2 hr after dosing and that of glycolate between 4-6 hr. The rate of ethylene glycol elimination was somewhat faster in rats with a half-life of 1.7 hr compared to 3.4 hr in dogs. The maximum plasma level of glycolate was greater in rats although the pattern of accumulation was similar to that in dogs. Glycolate disappeared from the plasma at the same time as ethylene glycol, suggesting a slower rate of elimination of the metabolite than that of ethylene glycol. Renal excretion of ethylene glycol was an important route for its elimination accounting for 20-30% of the dose. Renal excretion of glycolate represented about 5% of the dose. Ethylene glycol induced an immediate, but short lived diuresis compared to that in control rats. Minimal clinical effects (mild acidosis with no sedation) were noted at these doses of ethylene glycol (1-2 g/kg) in both rats and dogs. The results indicate that the toxicokinetics of ethylene glycol and glycolate were similar in both species. The effect of 0.35 to 0.8 mmol/kg glycolic acid and 1.0 to 4.4 mmol/kg sodium glycolate on cyclopropane-epinephrine induced cardiac arrhythmias was examined using dogs. Doses of 0.35 to 0.5 mmol/kg glycolic acid increased the duration of arrhythmias in the 13 dogs tested, whereas doses >0.5 mmol/kg decreased or totally eliminated the arrhythmias in each of 11 dogs. Depression was observed for many of the dogs at higher doses. Sodium glycolate was much less effective in decreasing the arrhythmias, with 3 mmol/kg being required and its action being transient. Therapeutic Uses Keratolytic Agents Glycolic acid is a member of the alpha-hydroxy acid (AHA) family, which ... has been used for centuries as a cutaneous rejuvenation treatment. Recently it has proved to be a versatile peeling agent and it is now widely used to treat many defects of the epidermis and papillary dermis in a variety of strengths, ranging from 20% to 70%, depending on the condition being treated. People of almost any skin type and color are candidates, and almost any area of the body can be peeled... Glycolic acid has been used by dermatologists for years to treat skin disorders and is a component of many over-the-counter personal care products. No systemic toxicity has been noted as a result of these uses. Chemical peeling, also known as chemoexfoliation or dermapeeling, is performed to improve the skin's appearance as it reduces the wrinkles caused by aging and the features of photoaged skin. Although the best results are obtained with deep /phenol/ peels, the medium-depth peels allow to obtain excellent results without the dangerous side effects of deep peels. Medium-depth peelings are performed with trichloroacetic acid (TCA) at 35-50% alone or at 35% in combination with Jessner's solution, 70% glycolic acid, and solid CO(2)... For more Therapeutic Uses (Complete) data for HYDROXYACETIC ACID (27 total), please visit the HSDB record page. Drug Warnings FDA has considered evidence that suggests that topically applied cosmetic products containing alpha hydroxy acids (AHAs) as ingredients may increase the sensitivity of skin to the sun while the products are used and for up to a week after use is stopped, and that this increased skin sensitivity to the sun may increase the possibility of sunburn. ... As an interim measure, while FDA continues to review the data on AHAs to address the potential for this increased skin sensitivity to the sun, FDA is recommending that the labeling of a cosmetic product that contains an AHA as an ingredient and that is topically applied to the skin or mucous membrane bear a statement that conveys the following information. The information in the AHA labeling statement is consistent with FDA's current thinking on sun protection. Sunburn Alert: This product contains an alpha hydroxy acid (AHA) that may increase your skin's sensitivity to the sun and particularly the possibility of sunburn. Use a sunscreen, wear protective clothing, and limit sun exposure while using this product and for a week afterwards. /Alpha hydroxy acids/ 1989-1996 Consumer adverse experience reports that were submitted to FDA headquarters and to FDA district offices on alpha hydroxy acid (AHA)-containing products /were evaluated/. Typical adverse reactions included "severe redness, swelling (especially in the area of the eyes), burning, blistering, bleeding, scarring, rash, itching, contact dermatitis, skin discoloration (reportedly permanent), and adverse neurological responses." Some of the individuals submitting an adverse experience report were seen by a physician, and at least one adverse report involved professional application and at least one involved a product prescribed by a dermatologist. FDA's submittal stated that "in addition to consumer reports of adverse reactions, letters have also been received from dermatologists treating patients suffering from injuries resulting from the use of these (AHA-containing) products". /Alpha hydroxy acids/ |
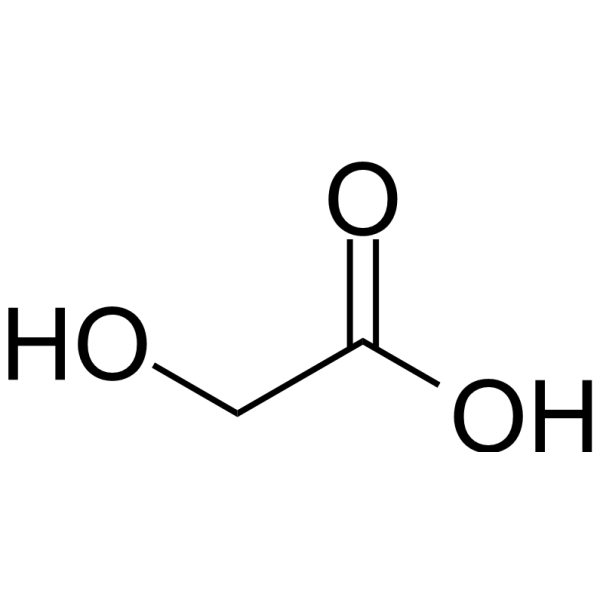
| 分子式 |
C2H4O3
|
|---|---|
| 分子量 |
76.05
|
| 精确质量 |
76.016
|
| CAS号 |
79-14-1
|
| 相关CAS号 |
Glycolic acid-d2;75502-10-2;Glycolic acid-13C2;111389-68-5
|
| PubChem CID |
757
|
| 外观&性状 |
Colorless, translucent solid
Solid glycolic acid forms colorless, monoclinic, prismatic crystals. Orthorhombic needles from water; leaves from diethyl ether |
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
265.6±13.0 °C at 760 mmHg
|
| 熔点 |
75-80 °C(lit.)
|
| 闪点 |
128.7±16.3 °C
|
| 蒸汽压 |
0.0±1.2 mmHg at 25°C
|
| 折射率 |
1.450
|
| LogP |
-1.05
|
| tPSA |
57.53
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
5
|
| 分子复杂度/Complexity |
40.2
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=C(CO)O
|
| InChi Key |
AEMRFAOFKBGASW-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C2H4O3/c3-1-2(4)5/h3H,1H2,(H,4,5)
|
| 化学名 |
2-hydroxyacetic acid
|
| 别名 |
Glycolic acid; Hydroxyethanoic acid; Hydroxyacetic acid
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ~100 mg/mL (~1314.92 mM)
DMSO : ≥ 100 mg/mL (~1314.92 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (32.87 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (32.87 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (32.87 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 100 mg/mL (1314.92 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 13.1492 mL | 65.7462 mL | 131.4924 mL | |
| 5 mM | 2.6298 mL | 13.1492 mL | 26.2985 mL | |
| 10 mM | 1.3149 mL | 6.5746 mL | 13.1492 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。