| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg | |||
| Other Sizes |
| 靶点 |
β-catenin-responsive transcription (CRT)
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
iCRT3 抑制对 Wnt 和 β-catenin 敏感的转录。 iCRT3 显着降低了 TOP Flash 活动和 NTSR1 级别。 iCRT3可以显着抵消神经降压素(NTS)和Wnt3a的抗凋亡作用[1]。与 DMSO 对照相比,长期 iCRT3 维持的细胞表现出经典多能性的表达增加,但同时分化标记物和 T 细胞因子 (TCF) 靶基因的表达减少[2]。 iCRT3 治疗在 12.5、25、50 和 75 μM 剂量下,TNF-α 水平分别降低 14.7%、18.5%、44.9% 和 61.3%。与媒介物相比,iCRT3 疗法的 IκB 水平呈剂量依赖性上升[3]。
|
||
| 体内研究 (In Vivo) |
iCRT3 治疗可显着降低肿瘤生长速度。 iCRT3 的肿瘤抑制功能始终与增殖标志物 Ki67 指数的下降相关[1]。与载体组相比,10 mg/kg iCRT3 治疗组的 IL-6 水平降低了 82.9%。在假手术中,检测不到 IL-1β 水平;然而,在脓毒症小鼠中,当给予 5 和 10 mg/kg iCRT3 时,它们分别达到 371 pg/mL 和下降 30.2% 和 53.2%。用 5 和 10 mg/kg 剂量的 iCRT3 治疗的这些脓毒症小鼠的 AST 水平分别比用载体治疗的动物低 15.4% 和 44.2%。与媒介物组相比,用 10 mg/kg iCRT3 治疗后,肺形态得到改善,微观退化更少。将 iCRT3 治疗动物的肺组织与媒介物组进行比较时,凋亡细胞减少了 92.7%[3]。
|
||
| 酶活实验 |
β-catenin-TCF报告活性测定[3]
RAW264.7细胞在转染前一天以1.24 × 105个细胞/ ml的密度接种。细胞与250 ng TOP-TK-Luc或TOP-TK-Luc和25 ng pRL-TK报告质粒短暂共转染,使用Lipofectamine 3000 Reagent,按照制造商的说明。转染24 h后,用iCRT3或对照物预处理细胞50 min,然后用LPS (1 ng/ml)刺激24 h。转染后48小时裂解细胞,根据制造商的说明,用双荧光素酶报告基因检测系统测定荧光素酶活性。TOP-TK-Luc包含最优位点,TOP-TK-Luc包含位于萤火虫荧光素酶报告基因上游的突变tcf结合位点。将TOP和FOP萤火虫荧光素酶活性归一化为来自共转染pRL-TK质粒的Renilla荧光素酶活性,作为转染效率的内部对照。所有实验都进行了至少两次的三次重复。 |
||
| 细胞实验 |
荧光素酶报告试验[1]
细胞在24孔板中以4 × 105个细胞/孔的大小进行镀膜,用Lipofectamine 2000 瞬时转染TopFlash(0.5µg)和Renilla报告基因(0.05µg)。A172或U87细胞中分别加入NTS、Wnt3a、SR48692和iCRT3处理24 h。收集细胞,转染2天后测定荧光素酶活性。采用双荧光素酶报告基因检测系统测定荧光素酶活性。 细胞增殖和细胞凋亡试验[1] 将细胞接种到96孔板中,每孔密度为5 × 103个细胞,在指定处理的培养基中再孵育48小时。根据制造商的说明,分别使用Cell Counting kit-8和Caspase-Glo 3/7检测试剂盒进行细胞活力和细胞凋亡检测。 对于长期培养,在DMSO或iCRT3的干条件下(血清加LIF),将细胞以有限的稀释度在6孔或96孔板中进行多次传代(14 d),每天更换培养基。每代进行AP染色以监测相对多能性水平。使用的小分子包括10µM iCRT3和1µM XAV939,用DMSO稀释。L- wnt3a和对照L细胞是R.T. Moon赠送的。[2] |
||
| 动物实验 |
|
||
| 参考文献 |
|
||
| 其他信息 |
Background/aims: Neurotensin (NTS), an intestinal hormone, is profoundly implicated in cancer progression through binding its primary receptor NTSR1. The conserved Wnt/β-Catenin pathway regulates cell proliferation and differentiation via activation of the β-catenin/T-cell factor (TCF) complex and subsequent modulation of a set of target genes. In this study, we aimed to uncover the potential connection between NTS/NTSR1 signaling and Wnt/β-Catenin pathway.
Methods: Genetic silencing, pharmacological inhibition and gain-of-function studies as well as bioinformatic analysis were performed to uncover the link between NTS/ NTSR1 signaling and Wnt/β-Catenin pathway. Two inhibitors were used in vivo to evaluate the efficiency of targeting NTS/NTSR1 signaling or Wnt/β-Catenin pathway.
Results: We found that NTS/NTSR1 induced the activation of mitogen-activated protein kinase (MAPK) and the NF-κB pathway, which further promoted the expression of Wnt proteins, including Wnt1, Wnt3a and Wnt5a. Meanwhile, the mRNA and protein expression levels of NTSR1 were increased by the Wnt pathway activator Wnt3a and decreased by the Wnt inhibitor iCRT3 in glioblastoma cells. Furthermore, pharmacological inhibition of NTS/NTSR1 or Wnt/β-Catenin signaling suppressed tumor growth in vitro and in vivo.
Conclusion: These results reveal a positive feedback loop between NTS/NTSR1 and Wnt/β-Catenin signaling in glioblastoma cells that might be important for tumor development and provide potential therapeutic targets for glioblastoma.[1]
The ability of mouse embryonic stem cells (mESCs) to self-renew or differentiate into various cell lineages is regulated by signaling pathways and a core pluripotency transcriptional network (PTN) comprising Nanog, Oct4, and Sox2. The Wnt/β-catenin pathway promotes pluripotency by alleviating T cell factor TCF3-mediated repression of the PTN. However, it has remained unclear how β-catenin's function as a transcriptional activator with TCF1 influences mESC fate. Here, we show that TCF1-mediated transcription is up-regulated in differentiating mESCs and that chemical inhibition of β-catenin/TCF1 interaction improves long-term self-renewal and enhances functional pluripotency. Genetic loss of TCF1 inhibited differentiation by delaying exit from pluripotency and conferred a transcriptional profile strikingly reminiscent of self-renewing mESCs with high Nanog expression. Together, our data suggest that β-catenin's function in regulating mESCs is highly context specific and that its interaction with TCF1 promotes differentiation, further highlighting the need for understanding how its individual protein-protein interactions drive stem cell fate. [2] The Wnt/β-catenin pathway has been involved in regulating inflammation in various infectious and inflammatory diseases. Sepsis is a life-threatening condition caused by dysregulated inflammatory response to infection with no effective therapy available. Recently elevated Wnt/β-catenin signaling has been detected in sepsis. However, its contribution to sepsis-associated inflammatory response remains to be explored. In this study, we show that inhibition of Wnt/β-catenin signaling reduces inflammation and mitigates sepsis-induced organ injury. Using in vitro LPS-stimulated RAW264.7 macrophages, we demonstrate that a small-molecule inhibitor of β-catenin responsive transcription, iCRT3, significantly reduces the LPS-induced Wnt/β-catenin activity and also inhibits TNF-α production and IκB degradation in a dose-dependent manner. Intraperitoneal administration of iCRT3 to C57BL/6 mice, subjected to cecal ligation and puncture-induced sepsis, decreases the plasma levels of proinflammatory cytokines and organ injury markers in a dose-dependent manner. The histological integrity of the lungs is improved with iCRT3 treatment, along with reduced lung collagen deposition and apoptosis. In addition, iCRT3 treatment also decreases the expression of the cytokines, neutrophil chemoattractants, as well as the MPO activity in the lungs of septic mice. Based on these findings we conclude that targeting the Wnt/β-Catenin pathway may provide a potential therapeutic approach for treatment of sepsis.[3] |
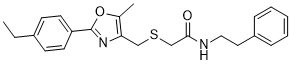
| 分子式 |
C23H26N2O2S
|
|
|---|---|---|
| 分子量 |
394.53
|
|
| 精确质量 |
394.171
|
|
| 元素分析 |
C, 70.02; H, 6.64; N, 7.10; O, 8.11; S, 8.13
|
|
| CAS号 |
901751-47-1
|
|
| 相关CAS号 |
|
|
| PubChem CID |
6622273
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| LogP |
5.195
|
|
| tPSA |
80.43
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
4
|
|
| 可旋转键数目(RBC) |
9
|
|
| 重原子数目 |
28
|
|
| 分子复杂度/Complexity |
462
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
QTDYVSIBWGVBKU-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C23H26N2O2S/c1-3-18-9-11-20(12-10-18)23-25-21(17(2)27-23)15-28-16-22(26)24-14-13-19-7-5-4-6-8-19/h4-12H,3,13-16H2,1-2H3,(H,24,26)
|
|
| 化学名 |
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.34 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.34 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5347 mL | 12.6733 mL | 25.3466 mL | |
| 5 mM | 0.5069 mL | 2.5347 mL | 5.0693 mL | |
| 10 mM | 0.2535 mL | 1.2673 mL | 2.5347 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Proc Natl Acad Sci U S A.2011 Apr 12;108(15):5954-63. |
|---|
Proc Natl Acad Sci U S A.2011 Apr 12;108(15):5954-63. |
Proc Natl Acad Sci U S A.2011 Apr 12;108(15):5954-63. |
Effect of iCRT3 administration on systemic cytokine levels after CLP.Sci Rep.2017 Aug 23;7(1):9235. |
|---|
Effect of iCRT3 treatment on the expression of cytokines in the lungs after CLP.Sci Rep.2017 Aug 23;7(1):9235. |
Effect of iCRT3 treatment on the neutrophil infiltration in the lungs after CLP.Sci Rep.2017 Aug 23;7(1):9235. |