| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
FGFR1 (IC50 = 0.9 nM); FGFR2 (IC50 = 1.4 nM); FGFR3 (IC50 = 1 nM); FGFR4 (IC50 = 60 nM)
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:BGJ398 还可以抑制 VEGFR2,但效力较低。 BGJ398 抑制 VEGFR2 的 IC50 为 0.18 μM。 BGJ398 抑制其他激酶,包括 ABL、FYN、KIT、LCK、LYN 和 YES,IC50 分别为 2.3 μM、1.9 μM、0.75 μM、2.5 μM、0.3 μM 和 1.1 μM。在细胞水平上,BGJ398 抑制 FGFR1-、FGFR2-Q 和 FGFR3 依赖性 BaF3 细胞的增殖,IC50 分别为 2.9 μM、2.0 μM 和 2 μM。 BGJ398 干扰特定酪氨酸残基的自磷酸化,包括 FGFR-WT、FGFR2-WT、FGFR3-K650E、FGFR3-S249C 和 FGFR4-WT,IC50 分别为 4.6 nM、4.9 nM、5 nM、5 nM 和 168 nM。 BGJ398 抑制野生型 (WT) FGFR3 过表达的癌细胞(例如 RT112、RT4、SW780 和 JMSU1)的增殖,IC50 分别为 5 nM、30 nM、32 nM 和 15 nM。激酶测定:在放射性标记的 ATP 存在下,通过测量纯化的 GST 融合 FGFR3-K650E 激酶结构域对合成底物的磷酸化来评估酶促激酶活性。通过将 10 μL 3 倍浓缩的 BGJ398 溶液或对照与 10 μL 相应底物混合物(肽底物、ATP 和 [γ33P]ATP)混合来测量酶活性。通过添加 10 μL 3 倍浓缩的酶溶液(在测定缓冲液中)来启动反应。测定组分的最终浓度如下:10 ng GST-FGFR3-K650E、20 mM Tris-HCl、pH 7.5、3 mM MnCl2、3 mM MgCl2、1 mM DTT、250 μg/mL PEG 20000、2 μg /mL 聚 (EY) 4:1、1% DMSO 和 0.5 μM ATP (γ-[33P]-ATP 0.1 μCi)。根据过滤结合 (FB) 方法,在 96 孔板中在室温下进行 10 分钟,最终体积为 30 μL,包括 BGJ398。通过添加 20 μL 125 mM EDTA 来终止酶反应,并按如下方式对 33P 掺入多肽底物进行定量:将 30 μL 停止的反应混合物转移到之前用 125 mM EDTA 浸泡 5 分钟的 Immobilon-PVDF 膜上。甲醇,用水冲洗,用 0.5% H3PO4 浸泡 5 分钟,然后安装在断开真空源的真空歧管上。点样后,连接真空,并用 0.5% H3PO4 (200 μL) 冲洗每个孔。除去游离膜并在摇床上用 1% H3PO4 抛光四次,用乙醇抛光一次。将膜干燥并添加 10 μL/孔的闪烁液覆盖。最终将板密封并在微板闪烁计数器中计数。 IC50值通过BGJ398的抑制百分比的线性回归分析来计算。细胞测定:鼠 BaF3 细胞系在补充有 10% FBS 的 RPMI-1640 培养基中培养,通过使用通过突变或与二聚化伙伴融合激活的酪氨酸激酶进行稳定转导,使其增殖和存活不依赖于 IL-3。 4.5 g/L 葡萄糖、1.5 g/L 碳酸氢钠和青霉素/链球菌。细胞每周传代两次。使用荧光素酶生物发光测定评估 BGJ398 介导的 BaF3 细胞增殖和活力的抑制。使用 μFill 液体分配器将指数生长的 BaF3 或 BaF3 Tel-TK 细胞以 50 μL/孔接种到 384 孔板(4250 个细胞/孔)中。 BGJ398 在 DMSO 中连续稀释并排列在聚丙烯 384 孔板中。然后使用 pintool 转移装置将 50 nL BGJ398 转移至含有细胞的板中,并将板在 37 °C (5% CO2) 下孵育 48 小时。然后添加 25 μL Bright-Glo,并使用 Analyst-GT 定量发光。使用定制曲线拟合软件来生成细胞活力百分比与抑制剂浓度对数函数的逻辑拟合。 IC50 值确定为将细胞活力降低至 DMSO 对照的 50% 所需的 BGJ398 浓度。
|
| 体内研究 (In Vivo) |
在该原位异种移植膀胱癌模型中,连续 12 天口服 10 和 30 mg/kg 剂量的 BGJ398 后可诱导肿瘤生长抑制和停滞。有趣的是,接受 BGJ398 的动物要么没有体重减轻(10 毫克/公斤),要么体重增加 10%(30 毫克/公斤),这进一步表明了疗效。 RT112荷瘤雌性Rowett大鼠接受单次口服BGJ398单磷酸盐,剂量为4.25和8.51 mg/kg。 BGJ398 以剂量依赖性方式显着降低 pFRS2 和 pMAPK 的水平。 BGJ398 以剂量依赖性方式显着抑制 bFGF 刺激的血管生成。然而,BGJ398 不会损害 VEGF 诱导的血管形成。
|
| 酶活实验 |
纯化的 GST 融合 FGFR3-K650E 激酶结构域在放射性标记 ATP 存在的情况下磷酸化合成底物,以测量酶激酶活性。通过将 10 μL 相应底物混合物(肽底物、ATP 和 [γ33P]ATP)与 10 μL 3 倍浓缩的 Infigratinib 溶液或对照混合来测定酶活性。将测定缓冲液与 10 μL 浓缩酶溶液混合 3 次以启动反应。以下是检测成分的最终浓度:0.5 μM ATP (γ-[33P]-ATP 0.1 μCi)、3 mM MnCl2、3 mM MgCl
|
| 细胞实验 |
添加有 10% FBS、4.5 g/L 葡萄糖、1.5 g/L 碳酸氢钠和 Pen/Strep 的 RPMI-1640 培养基用于培养小鼠 BaF3 细胞系。每周两次细胞通过。荧光素酶生物发光测定用于评估化合物介导的 BaF3 细胞增殖和活力的抑制。使用 μFill 液体分配器,将指数生长的 BaF3 或 BaF3 Tel-TK 细胞以 50 μL/孔接种到新鲜培养基中的 384 孔板(4250 个细胞/孔)中。在 DMSO 中连续稀释后,将 infigratinib 置于 384 孔聚丙烯板中。使用 pintool 转移装置将 50 nL 化合物转移至板中后,将板在 37°C (5% CO 2 ) 下孵育 48 小时。接下来,添加 25 μL Bright-Glo,并使用 Analyst-GT 测量发光。使用专门的曲线拟合软件生成细胞活力百分比与抑制剂浓度对数函数的逻辑拟合。将细胞活力降低至 DMSO 对照的 50% 所需的化合物浓度称为 IC50 值[1]。
|
| 动物实验 |
Mice: HsdNpa female: Athymic Nude-nu mice are employed. Infigratinib is taken orally for 12 straight days at doses of 10 and 30 mg/kg/qd. It is prepared as a suspension in PEG300/D5W (2:1, v/v). ANOVA is used to analyze the tumor and body weight data, and Dunnett's test is used to compare the treatment group to the control group post hoc. For intragroup comparison, the post hoc Tukey test is employed. To perform statistical analysis, use GraphPad Prism 4.02. One calculates the T/C (%) value as a measure of efficacy.
Rats: Woman in the nude 6 to 9-week-old Rowett rats are utilized. The tumor-bearing rats (n=8) receive infigratinib intraperitoneally (gavage) once a day for 20 days at doses of 5, 10, and 15 mg/kg/qd (free base equivalents). The drug is prepared as a solution in acetic acid-acetate buffer pH 4.6/PEG300 (1:1, v/v). It uses five milliliters per kilogram. The formula for determining tumor volumes is length×width×height×π/6, which can be measured using calipers. Antitumor activity is represented as T/C (%), which is calculated as (mean change in tumor volume of treated animals / mean change in tumor volume of control animals)×100. One calculates regressions (%). |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Absorption Mean (%CV) Cmax is 282.5 ng/mL (54%) and AUC0-24h is 3780 ngxh/mL (59%) for infigratinib. Infigratinib Cmax and AUC increase more than proportionally across the dose range of 5 to 150 mg and steady state is achieved within 15 days. At steady state, median time to achieve peak infigratinib plasma concentration (Tmax) is six hours, with a range between two and seven hours. Mean (%CV) Cmax is 42.1 ng/mL (65%) for BHS697 and 15.7 ng/mL (92%) for CQM157. Mean (%CV) AUC0-24h is 717 ngxh/mL (55%) for BHS697 and 428 ngxh/mL (72%) for CQM157. In healthy subjects, a high-fat and high-calorie meal increased AUCinf of infigratinib by 80%-120% and Cmax by 60%-80%. The median Tmax also shifted from four hours to six hours. A low-fat low-calorie meal increased the mean AUCinf of infigratinib by 70% and Cmax by 90%/ Route of Elimination Following administration of a single oral dose of radiolabeled infigratinib in healthy subjects, approximately 77% of the dose was recovered in feces, where 3.4% of the dose was in the unchanged parent form. About 7.2% was recovered in urine with 1.9% of the dose was unchanged. Volume of Distribution At steady state, the geometric mean (CV%) apparent volume of distribution of infigratinib was 1600 L (33%). In rats receiving a single oral dose, infigratinib had brain-to-plasma concentration ratios (based on AUC0-inf) of 0.682. Clearance The geometric mean (CV%) total apparent clearance (CL/F) of infigratinib was 33.1 L/h (59%) at steady state. Metabolism / Metabolites According to _in vitro_ findings, about 94% of infigratinib is metabolized by CYP3A4 and about 6% of the drug is metabolized by flavin-containing monooxygenase 3 (FMO3). About 38% of the dose is circulating parent drug in the plasma and BHS697 and CQM157 are two major metabolites of infigratinib that are each found at >10% of the dose. They are pharmacologically active, with BHS697 representing about 16% to 33% of the overall pharmacological activity of infigratinib and CQM157 contributing to about 9% to 12%. BHS697 undergoes further metabolism mediated by CYP3A4 and CQM157 is metabolized through both Phase I and Phase II biotransformation pathways. The exact metabolic pathways and the structure of BHS697 and CQM157 are not fully characterized. Biological Half-Life The geometric mean (CV%) terminal half-life of infigratinib was 33.5 h (39%) at steady state. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In the open label clinical trials of infigratinib for advanced or metastatic cholangiocarcinoma, adverse events were common and led to dose interruptions in 64%, dose reductions in 60% of patients, and permanent discontinuations in 15% largely for hyperphosphatemia, infections and sepsis rather than liver injury. In preregistration trials in 108 patients, ALT elevations arose in 51% and to above 5 times ULN in 6%. The elevations were typically self-limited and resolved rapidly with or without dose adjustments. No patients developed clinically apparent liver injury or jaundice. Since its approval, there have been no reports clinically apparent liver injury attributed to infigratinib. However, the total clinical experience with its use has been limited and the frequency of serum aminotransferase elevations during therapy suggest that clinically significant liver injury may occur. Likelihood score: E* (unproven but possible, rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Infigratinib is no longer marketed in the US. No information is available on the clinical use of infigratinib during breastfeeding. Because infigratinib is 96.8% bound to plasma proteins, the amount in milk is likely to be low. However, because of its potential toxicity in the breastfed infant and its half-life of 33.5 hours, the manufacturer recommends that breastfeeding be discontinued during infigratinib therapy and for 1 month after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Infigratinib is about 96.8% bound to plasma proteins, primarily to lipoprotein. Protein binding is concentration-dependent. |
| 参考文献 |
|
| 其他信息 |
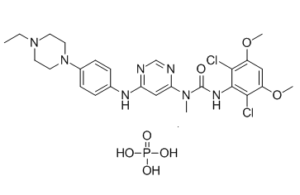
Infigratinib Phosphate is the phosphate salt form of infigratinib, an orally bioavailable pan-inhibitor of human fibroblast growth factor receptors (FGFRs) with potential antiangiogenic and antineoplastic activities. Upon administration, infigratinib selectively binds to and inhibits the activities of FGFRs, which may result in the inhibition of angiogenesis and cell proliferation, and the induction of cell death in tumors with activating FGFR amplifications, mutations, or fusions. FGFRs are a family of receptor tyrosine kinases that are involved in tumor cell differentiation and proliferation, tumor angiogenesis, and tumor cell survival. Activating FGFR amplifications, mutations, or fusions occur in various cancer cell types.
See also: Infigratinib (has active moiety). A novel series of N-aryl-N'-pyrimidin-4-yl ureas has been optimized to afford potent and selective inhibitors of the fibroblast growth factor receptor tyrosine kinases 1, 2, and 3 by rationally designing the substitution pattern of the aryl ring. On the basis of its in vitro profile, compound 1h (NVP-BGJ398) was selected for in vivo evaluation and showed significant antitumor activity in RT112 bladder cancer xenografts models overexpressing wild-type FGFR3. These results support the potential therapeutic use of 1h as a new anticancer agent.[1] The recent identification of activating fibroblast growth factor receptor 2 (FGFR2) mutations in endometrial cancer has generated an opportunity for a novel target-based therapy. Here, we explore the therapeutic potential of 2 FGFR inhibitors, the multikinase inhibitor dovitinib (TKI258) and the more selective FGFR inhibitor NVP-BGJ398 for the treatment of endometrial cancer. We examined the effects of both inhibitors on tumor cell growth, FGFR2 signaling, cell cycle, and apoptosis using a panel of 20 molecularly characterized human endometrial cancer cell lines. Anchorage-independent growth was studied using soft agar assays. In vivo studies were conducted using endometrial cancer xenograft models. Cell lines with activating FGFR2 mutations (S252W, N550K) were more sensitive to dovitinib or NVP-BGJ398 when compared with their FGFR2 wild-type counterparts (P = 0.073 and P = 0.021, respectively). Both agents inhibited FGFR2 signaling, induced cell-cycle arrest, and significantly increased apoptosis in FGFR2-mutant lines. In vitro, dovitinib and NVP-BGJ398 were both potent at inhibiting cell growth of FGFR2-mutant endometrial cancer cells, but the activity of dovitinib was less restricted to FGFR2-mutant lines when compared with NVP-BGJ398. In vivo, dovitinib and NVP-BGJ398 significantly inhibited the growth of FGFR2-mutated endometrial cancer xenograft models. In addition, dovitinib showed significant antitumor activity in FGFR2 wild-type endometrial cancer xenograft models including complete tumor regressions in a long-term in vivo study. Dovitinib and NVP-BGJ398 warrant further clinical evaluation in patients with FGFR2-mutated endometrial cancer. Dovitinib may have antitumor activity in endometrial cancer beyond FGFR2-mutated cases and may permit greater flexibility in patient selection.[2] |
| 分子式 |
C26H34CL2N7O7P
|
|
|---|---|---|
| 分子量 |
658.47
|
|
| 精确质量 |
657.163
|
|
| 元素分析 |
C, 47.43; H, 5.20; Cl, 10.77; N, 14.89; O, 17.01; P, 4.70
|
|
| CAS号 |
1310746-10-1
|
|
| 相关CAS号 |
Infigratinib;872511-34-7
|
|
| PubChem CID |
56669626
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| LogP |
4.574
|
|
| tPSA |
182.66
|
|
| 氢键供体(HBD)数目 |
5
|
|
| 氢键受体(HBA)数目 |
12
|
|
| 可旋转键数目(RBC) |
8
|
|
| 重原子数目 |
43
|
|
| 分子复杂度/Complexity |
773
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
ClC1C(=C([H])C(=C(C=1N([H])C(N(C([H])([H])[H])C1C([H])=C(N=C([H])N=1)N([H])C1C([H])=C([H])C(=C([H])C=1[H])N1C([H])([H])C([H])([H])N(C([H])([H])C([H])([H])[H])C([H])([H])C1([H])[H])=O)Cl)OC([H])([H])[H])OC([H])([H])[H].P(=O)(O[H])(O[H])O[H]
|
|
| InChi Key |
GUQNHCGYHLSITB-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C26H31Cl2N7O3.H3O4P/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28;1-5(2,3)4/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31);(H3,1,2,3,4)
|
|
| 化学名 |
3-(2,6-dichloro-3,5-dimethoxyphenyl)-1-[6-[4-(4-ethylpiperazin-1-yl)anilino]pyrimidin-4-yl]-1-methylurea;phosphoric acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|---|
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5187 mL | 7.5934 mL | 15.1867 mL | |
| 5 mM | 0.3037 mL | 1.5187 mL | 3.0373 mL | |
| 10 mM | 0.1519 mL | 0.7593 mL | 1.5187 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05514912 | Not yet recruiting | Drug: Cisplatin Drug: Infigratinib Phosphate |
Stage 0 Intrahepatic Cholangiocarcinoma AJCC v8 Resectable Intrahepatic Cholangiocarcinoma |
Emory University | November 1, 2023 | Phase 2 |
| NCT04197986 | Terminated | Drug: Infigratinib Drug: Placebo |
Upper Tract Urothelial Carcinomas Urothelial Bladder Cancer |
QED Therapeutics, Inc. | March 11, 2020 | Phase 3 |
Targeting Fgfr2-fusion containing tumors with the FGFR-inhibitor BGJ398 results in complete response.Cancer Discov.2018 Mar;8(3):354-369. |
Multiple, different genetic aberrations lead to common elevated MAPK and/or PI3K pathway activation in human breast cancer patients.Cancer Discov.2018 Mar;8(3):354-369. |
Targeting Dhx9-Raf1 and cMet with MEK- and MET-inhibitor, respectively, result in tumor regression or delayed progression.
|