| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
CaMKII (calmodulin-dependent kinase type II) (Ki = 370 nM)
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
用 KN-93 盐酸盐处理两天后,95% 的细胞处于 G1 期。 G1 停滞是可逆的,KN-93 盐酸盐释放一天后,细胞达到 S 期和 G2-M 期高峰。 KN-93 盐酸盐还抑制 NIH 3T3 成纤维细胞响应碱性成纤维细胞生长因子、血小板衍生生长因子-BB 和表皮生长因子的增殖 [1]。虽然 KN-93 盐酸盐强烈消散胃膜囊泡中产生的质子梯度并减少腔体积,但它抑制 H+ 和 K+-ATP 酶的作用 [2]。 KN-93 盐酸盐 (0.5 μM) 可抑制早期后除极和动作电位延长期间左心室应力升高的发展。早期后除极的特点是独立于 Ca2+ 的 CaM 激酶活性升高,该活性可被 KN-93 盐酸盐抑制 [3]。
|
||
| 体内研究 (In Vivo) |
KN-93 (5 μg) 通过降低帕金森病大鼠模型中 pGluR1S845 的表达来改善左旋多巴诱导的运动障碍。在 MRL/lpr Foxp3-GFP 小鼠中,KN-93 可显着诱导脾脏、外周淋巴结和外周血中的 Treg 细胞,并减少皮肤和肾脏损伤。
KN-93(1 mg/kg/天,腹膜内注射)可降低糖尿病视网膜中 CaMKII 和 NF-κB 的磷酸化,并抑制糖尿病引起的视网膜血管渗漏[4]。
多功能钙/钙调素依赖性蛋白激酶II(CaM激酶)介导钙诱导的L型钙电流(ICa)增加;因此,它可能在ICa引起的早期后去极化(EADs)期间充当致心律失常信号分子。为了研究钙调素激酶激活对ICa依赖性EADs有利的假设,在离体兔心脏中用clofilium诱导EADs。所有EAD均迅速用ICa拮抗剂终止。在接触氯菲林之前,用钙调素激酶抑制剂KN-93或非活性类似物KN-92(0.5微M)预处理心脏10分钟。与KN-92(EAD存在于10/11颗心脏中)相比,KN-93(EAD在4/10颗心脏中存在)显著抑制了EAD(P=0.024)。在KN-93或KN-92治疗的心脏中,单相动作电位持续时间或心率等有利于EAD的参数没有显著差异。与无EAD的心脏相比,EAD心脏的CaM激酶原位活性增加了37%(P=0.015)。用KN-93预处理可以防止钙调素激酶活性的增加。在体外,KN-93有效地抑制了兔心肌CaM激酶活性(计算Ki100微M)。KN-93和KN-92对ICa和其他复极K+电流的作用并不能解释KN-93对EAD的优先抑制。这些数据显示了钙调素激酶活化与EAD之间的新关联,并与ICa和钙调素酶活化都有助于该模型中EAD的假设一致。[3] 姜黄素和KN-93抑制糖尿病引起的视网膜血管渗漏[4] 伊文思蓝用于视网膜平板支架,以评估姜黄素对视网膜血管渗漏的影响。在对照视网膜中,伊文思蓝荧光位于血管内(图2A)。在STZ治疗的大鼠中,观察到染料从毛细血管和较大血管的局灶性泄漏(图2B,箭头),这与其他报告一致。在服用姜黄素(图2C)或KN93(图2D)的STZ治疗大鼠中没有发现这种泄漏。测量视网膜中的伊文思蓝以评估BRB通透性(图2E)。与对照组动物(0.82±0.11μg Evans蓝/g湿重视网膜)相比,STZ治疗的糖尿病大鼠视网膜中的Evans蓝水平升高(2.89±0.47μg Evans-blue/g湿重视网膜。与全量成像显示的血管渗漏减少一致,在服用姜黄素(1.24±0.21μg)或KN93(1.37±0.35μg)的STZ治疗大鼠中,这种升高显著降低。 姜黄素和KN-93可降低糖尿病视网膜中VEGF、iNOS和ICAM-1的表达[4] 血管渗漏和白细胞粘附到视网膜血管是由促炎细胞因子介导的。因此,我们测定了姜黄素对VEGF、iNOS和ICAM-1表达水平的影响。与非糖尿病对照组相比,STZ治疗的糖尿病大鼠视网膜中的mRNA(图3A)和蛋白质(图3B,C)测量值显著升高。姜黄素或KN93的给药显著降低了这些增加。 姜黄素和KN-93抑制糖尿病视网膜中CaMKII和NF-κB的磷酸化[4] NF-κB p65亚基的磷酸化在调节许多基因的表达中起着重要作用,包括编码促炎细胞因子和粘附分子的基因。此外,CaMKII的磷酸化是糖尿病小鼠视网膜血管损伤发展的关键因素。为了评估姜黄素在调节CaMKII和NF-κB p65磷酸化中的作用,我们通过蛋白质印迹检测了视网膜。如图5所示,与对照组相比,STZ治疗的糖尿病大鼠视网膜中磷酸化CaMKII(Thr286)和NF-kB p65(Ser536)的水平显著升高。在给予姜黄素(100mg/kg/天)或KN93(1mg/kg/天)的STZ治疗的糖尿病大鼠中,这种升高是正常的。 2μg和5μgKN-93治疗均降低了左旋多巴引发PD大鼠的AIMs评分,但不影响左旋多巴的抗帕金森病作用。与行为分析一致,KN-93治疗(2μg)降低了PD大鼠的pGluR1S845水平。此外,KN-93治疗(2μg)降低了PD大鼠Gad1和Nur77的表达。 结论:这些数据表明,纹状体内注射KN-93有利于通过抑制PD大鼠CaMKII的激活来降低pGluR1S845的表达,从而降低LID的表达。pGluR1S845表达的降低进一步降低了PD大鼠Gad1和Nur77的表达[5]。 |
||
| 细胞实验 |
对于原代培养研究,如前所述,获得并鉴定了大鼠视网膜Müller细胞。简而言之,在出生后(PN)第5天至PN7天处死Sprague-Dawley大鼠,在无菌条件下清洗摘除的眼睛,丢弃前部。分离视网膜,切成1×1mm的碎片,在37°C下用0.1%胰蛋白酶处理20分钟,然后穿过网片去除任何大的视网膜碎片。将过滤的分离物以800rpm离心5分钟,并去除上清液。将沉淀的细胞重新悬浮并接种到含有添加了2 mmol/L谷氨酰胺、0.1%青霉素/链霉素和10%胎牛血清的Dulbecco改良Eagle培养基(DMEM)的塑料培养瓶中。培养物在37°C的5%二氧化碳中保持。每3-4天常规更换一次培养基。通过免疫细胞化学染色判断,Müller细胞通过谷氨酰胺合成酶(GS)和波形蛋白的表达进行鉴定。细胞核用DAPI(4',6-二脒基-2-苯基吲哚)染色。所有实验均使用80%-85%的融合细胞进行。每次实验前,将平板细胞与无血清DMEM培养基一起孵育1小时。之后,用无血清DMIM代替培养基,在有或没有10μmol/LKN-93、100μmol/L PDTC(吡咯烷二硫代氨基甲酸酯,一种NF-kB抑制剂)或指定浓度的姜黄素的情况下,用正常D-葡萄糖(5.5 mmol/L)或高葡萄糖(HG;30 mmol/L葡萄糖)处理细胞。[4]
细胞存活率评估:通过3-(4,5-二甲基噻唑-2-基)-2,5-二苯基溴化四唑(MTT)法评估细胞存活率。简而言之,将Müller细胞以每孔10×104个细胞的密度接种在96孔板上,并培养至亚融合。接下来,细胞用姜黄素处理24小时,然后在37°C、5%CO2气氛中用MTT(5 mg/mL)孵育4小时。然后去除培养基,将反应中形成的甲赞溶解在150μL DMSO(二甲基亚砜)中。使用多功能微孔板读数器在490nm处测量溶液的光密度。每个孔中的细胞存活率以对照组(载体处理组)的百分比表示。 |
||
| 动物实验 |
|
||
| 参考文献 |
|
||
| 其他信息 |
KN-93 is a sulfonamide resulting from the formal condensation of p-methoxybenzenesulfonic acid with the anilino nitrogen of 2-(aminomethyl)-N-(2-hydroxyethyl)aniline in which the hydrogens of the primary amino group have been replaced by methyl and p-chlorocinnamyl groups. KN-93 is a selective inhibitor of Ca(2+)/calmodulin-dependent protein kinase II. It has a role as an EC 2.7.11.17 (Ca(2+)/calmodulin-dependent protein kinase) inhibitor and a geroprotector. It is a sulfonamide, a tertiary amino compound, a primary alcohol, a member of monochlorobenzenes and a monomethoxybenzene.
Background: Levodopa remains the most effective drug for the treatment of Parkinson's disease (PD). However, long-term levodopa treatment is associated with the emergence of levodopa-induced dyskinesia (LID), which has hampered its use for PD treatment. The mechanisms of LID are only partially understood. A previous study showed that KN-93, a Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) inhibitor, could be used to ameliorate LID in rats. However, the precise mechanisms by which KN-93 acts as an antidyskinetic are not fully understood. Methods: In the present study, a rat model of PD was induced by 6-hydroxydopamine (OHDA) injections. Then, the successfully lesioned rats were intrastriatally administered with a different dose of KN-93 (1 μg, 2 μg, or 5 μg) prior to levodopa treatment. Abnormal involuntary movements (AIMs) scores and apomorphine-induced rotations were measured in PD rats. Phosphorylated levels of GluR1 at Serine-845 (pGluR1S845) levels were determined by western blot. Arc and Penk levels were measured by real-time polymerase chain reaction (PCR). Results: We found that both 2 μg and 5 μg KN-93 treatment lowered AIMs scores in levodopa priming PD rats without affecting the antiparkinsonian effect of levodopa. In agreement with behavioral analysis, KN-93 treatment (2 μg) reduced pGluR1S845 levels in PD rats. Moreover, KN-93 treatment (2 μg) reduced the expression of Gad1 and Nur77 in PD rats. Conclusion: These data indicated that intrastriatal injections of KN-93 were beneficial in reducing the expression of LID by lowering the expression of pGluR1S845 via suppressing the activation of CaMKII in PD rats. Decreased expression of pGluR1S845 further reduced the expression of Gad1 and Nur77 in PD rats.[5] Taken together, we found that intrastriatal administration of KN-93 (2 μg or 5 μg) reduced the expression of LID in levodopa primed PD rats. In addition, KN-93 treatment reduced pGluR1S845 levels and the expression of Gad1 and Nur77. We assume that KN-93 can ameliorate LID expression by reducing the expression of Gad1 and Nur77 which subsequently lowers the levels of pGluR1S845 in PD rats.[5] ackground: Curcumin possesses many pharmacological properties including anti-inflammatory effects. Although prior studies indicate that curcumin has beneficial effects for diabetic retinopathy, the mechanism of action is not known. To address this issue, we investigated the effect of curcumin against diabetes-induced retinal vascular damage and its mechanism of action by using cultured retinal Müller cells stimulated with high glucose. Methods: We studied the effects of curcumin in vivo in the retinas of rats rendered diabetic by streptozotocin and in vitro in Müller cells stimulated with high glucose. We administered curcumin, or KN93, an inhibitor of calcium/calmodulin dependent protein kinase II (CaMKII), or saline vehicle to experimental animals on a daily basis for 12 weeks. Primary cultures of rat Müller cells were incubated with normal glucose or high glucose, with or without curcumin, KN93, or pyrrolidine dithiocarbamate (PDTC), an inhibitor of the transcription protein nuclear factor κB (NF-κB). We examined mRNA and protein levels of vascular endothelial growth factor (VEGF), inducible nitric oxide synthase (iNOS) and intercellular adhesion molecule-1 (ICAM-1) by real-time RT-PCR and Western blotting, respectively. Retinal levels of CaMKII and NF-κB were examined by Western blotting. Vascular leakage was evaluated using Evans blue. Results: Curcumin and KN93 significantly inhibited the activation of CaMKII/NF-κB signaling induced by diabetes or elevated glucose, and subsequently decreased the expression of VEGF, iNOS and ICAM-1. These changes were associated with a decrease of diabetes-induced retinal vascular leakage. Conclusion: Curcumin protects the diabetic rat retina against early retinal vascular damage, by inhibition of CaMKII activity. Curcumin is currently used to treat a number of clinical conditions, and may prove beneficial for the management of diabetic retinopathy.[4] |
| 分子式 |
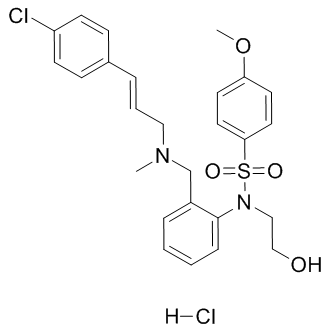
C26H30CL2N2O4S
|
|
|---|---|---|
| 分子量 |
537.5
|
|
| 精确质量 |
536.13
|
|
| 元素分析 |
C, 58.10; H, 5.63; Cl, 13.19; N, 5.21; O, 11.91; S, 5.96
|
|
| CAS号 |
1956426-56-4
|
|
| 相关CAS号 |
KN-93 hydrochloride;1956426-56-4;KN-93 phosphate;1913269-12-1; 1188890-41-6 (phosphate); 139298-40-1
|
|
| PubChem CID |
73425340
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| tPSA |
78.5
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
11
|
|
| 重原子数目 |
35
|
|
| 分子复杂度/Complexity |
713
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
CN(C/C=C/C1=CC=C(C=C1)Cl)CC2=CC=CC=C2N(CCO)S(=O)(=O)C3=CC=C(C=C3)OC.Cl
|
|
| InChi Key |
ATHMCQDBXQEIOK-IPZCTEOASA-N
|
|
| InChi Code |
InChI=1S/C26H29ClN2O4S.ClH/c1-28(17-5-6-21-9-11-23(27)12-10-21)20-22-7-3-4-8-26(22)29(18-19-30)34(31,32)25-15-13-24(33-2)14-16-25;/h3-16,30H,17-20H2,1-2H3;1H/b6-5+
|
|
| 化学名 |
N-[2-[N-(4-Chlorocinnamyl)-N-methylaminomethyl]phenyl]-N-(2-hydroxyethyl)-4-methoxybenzenesulfonamide hydrochloride
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.65 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.65 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.65 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8605 mL | 9.3023 mL | 18.6047 mL | |
| 5 mM | 0.3721 mL | 1.8605 mL | 3.7209 mL | |
| 10 mM | 0.1860 mL | 0.9302 mL | 1.8605 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。