| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
CRM1/chromosome region maintenance 1
|
|---|---|
| 体外研究 (In Vitro) |
KPT-185 使 MV4-11 和 OCI-AML3 细胞的细胞核中 CRM1 蛋白水平显着下降,p53 显着增加 [1]。 KPT-185(1-1000 nM;72 h)强烈降低 HPB-ALL、Jurkat、CCRF-CEM、MOLT-4、KOPTK1 和 LOUCY 细胞的增殖,IC50 为 16-395 nM [4]。 KPT-185 导致 MOLT-4 细胞系的细胞周期停滞在 G1 期 [4]。
|
| 体内研究 (In Vivo) |
最后,使用FLT3-ITD阳性MV4-11异种移植物小鼠模型,我们表明口服KPT-276(体内研究中KPT-185的类似物)治疗小鼠可显著延长白血病小鼠的存活时间(P<0.01)。总之,KPT-SINE在AML的体外和体内都非常有效[1]。
|
| 细胞实验 |
细胞活力测定[4]
细胞类型: HPB-ALL、Jurkat、CCRF-CEM、MOLT-4、KOPTK1、LOUCY 细胞 测试浓度: 1、10、100、1000 nM 孵育时间:72 小时 实验结果:这些细胞系的生长显着下降,IC50 为暴露 72 小时后为 16–395 nM。 |
| 动物实验 |
MV4-11 xenograft mouse model[1]
Spleen cells (0.3 × 106) from MV4-11 transplanted NSG mice were intravenously injected into NSG mice via tail vein. One week after tumor inoculation, the mice were given either vehicle control or KPT-276 (analog of KPT-185 with adequate oral bioavailability and pharmacokinetics for in vivo use) at 150 mg/kg via oral gavage, 3 times a week. Mice were monitored closely for clinical signs of leukemia, such as weight loss and hindlimb paralysis. Expected median survival for untreated animals in this model is 28 days. Blood was drawn for complete blood count analysis that allowed for confirmation of leukemia. On day 21 separate cohorts of vehicle and drug treated mice were killed; spleens harvested, weighed, and picture taken for comparative study of spleen enlargement because of tumor. Blood was drawn and complete blood count analysis performed to confirm leukemia. |
| 参考文献 |
|
| 其他信息 |
Chromosome maintenance protein 1 (CRM1) is a nuclear export receptor involved in the active transport of tumor suppressors (e.g., p53 and nucleophosmin) whose function is altered in cancer because of increased expression and overactive transport. Blocking CRM1-mediated nuclear export of such proteins is a novel therapeutic strategy to restore tumor suppressor function. Orally bioavailable selective inhibitors of nuclear export (SINE) that irreversibly bind to CRM1 and block the function of this protein have been recently developed. Here we investigated the antileukemic activity of KPT-SINE (KPT-185 and KPT-276) in vitro and in vivo in acute myeloid leukemia (AML). KPT-185 displayed potent antiproliferative properties at submicromolar concentrations (IC50 values; 100-500 nM), induced apoptosis (average 5-fold increase), cell-cycle arrest, and myeloid differentiation in AML cell lines and patient blasts. A strong down-regulation of the oncogene FLT3 after KPT treatment in both FLT3-ITD and wild-type cell lines was observed. Finally, using the FLT3-ITD-positive MV4-11 xenograft murine model, we show that treatment of mice with oral KPT-276 (analog of KPT-185 for in vivo studies) significantly prolongs survival of leukemic mice (P < .01). In summary, KPT-SINE are highly potent in vitro and in vivo in AML. The preclinical results reported here support clinical trials of KPT-SINE in AML.[1]
This study explored the anti-leukaemic efficacy of novel irreversible inhibitors of the major nuclear export receptor, chromosome region maintenance 1 (CRM1, also termed XPO1). We found that these novel CRM1 antagonists, termed SINE (Selective Inhibitors of Nuclear Export), induced rapid apoptosis at low nanomolar concentrations in a panel of 14 human T-cell acute lymphoblastic leukaemia (T-ALL) cell lines representing different molecular subtypes of the disease. To assess in vivo anti-leukaemia cell activity, we engrafted immunodeficient mice intravenously with the human T-ALL MOLT-4 cells, which harbour activating mutations of NOTCH1 and NRAS as well as loss of function of the CDKN2A, PTEN and TP53 tumour suppressors and express a high level of oncogenic transcription factor TAL1. Importantly, we examined the in vivo anti-leukaemic efficacy of the clinical SINE compound KPT-330 against T-ALL and acute myeloid leukaemia (AML) cells. These studies demonstrated striking in vivo activity of KPT-330 against T-ALL and AML cells, with little toxicity to normal murine haematopoietic cells. Taken together, our results show that SINE CRM1 antagonists represent promising 'first-in-class' drugs with a novel mechanism of action and wide therapeutic index, and imply that drugs of this class show promise for the targeted therapy of T-ALL and AML.[2] Overexpression of the cellular nuclear exportin 1, more commonly called chromosomal region maintenance 1 (CRM1), has been associated with malignant progression and mortality. Therefore, activation of nuclear export can play a significant etiologic role in some forms of human neoplasia and serve as a novel target for the treatment of these cancers. Mantle cell lymphoma (MCL) is an aggressive histotype of B-cell non-Hodgkin lymphoma that remains incurable. The objective of this study was to investigate the functional significance of CRM1 in MCL by evaluating the therapeutic efficacy of CRM1 inhibition in MCL in vitro and in vivo. Our results showed that CRM1 is highly expressed in MCL cells and is involved in regulating growth and survival mechanisms through the critical nuclear factor-κB survival pathway, which is independent of p53 status. Inhibition of CRM1 by two novel selective inhibitors of nuclear export (SINE), KPT-185 and KPT-276, in MCL cells resulted in significant growth inhibition and apoptosis induction. KPT-185 also induced CRM1 accumulation in the nucleus, resulting in CRM1 degradation by the proteasome. Oral administration of KPT-276 significantly suppressed tumor growth in an MCL-bearing severe combined immunodeficient mouse model, without severe toxicity. Our data suggest that SINE CRM1 antagonists are a potential novel therapy for patients with MCL, particular in relapsed/refractory disease.[3] Resistance to BRAF inhibitor therapy places priority on developing BRAF inhibitor-based combinations that will overcome de novo resistance and prevent the emergence of acquired mechanisms of resistance. The CRM1 receptor mediates the nuclear export of critical proteins required for melanoma proliferation, survival, and drug resistance. We hypothesize that by inhibiting CRM1-mediated nuclear export, we will alter the function of these proteins resulting in decreased melanoma viability and enhanced BRAF inhibitor antitumoral effects. To test our hypothesis, selective inhibitors of nuclear export (SINE) analogs KPT-185, KPT-251, KPT-276, and KPT-330 were used to induce CRM1 inhibition. Analogs PLX-4720 and PLX-4032 were used as BRAF inhibitors. Compounds were tested in xenograft and in vitro melanoma models. In vitro, we found CRM1 inhibition decreases melanoma cell proliferation independent of BRAF mutation status and synergistically enhances the effects of BRAF inhibition on BRAF-mutant melanoma by promoting cell-cycle arrest and apoptosis. In melanoma xenograft models, CRM1 inhibition reduces tumor growth independent of BRAF or NRAS status and induces complete regression of BRAF V600E tumors when combined with BRAF inhibition. Mechanistic studies show that CRM1 inhibition was associated with p53 stabilization and retinoblastoma protein (pRb) and survivin modulation. Furthermore, we found that BRAF inhibition abrogates extracellular signal-regulated kinase phosphorylation associated with CRM1 inhibition, which may contribute to the synergy of the combination. In conclusion, CRM1 inhibition impairs melanoma survival in both BRAF-mutant and wild-type melanoma. The combination of CRM1 and BRAF inhibition synergizes and induces melanoma regression in BRAF-mutant melanoma.[4] |
| 分子式 |
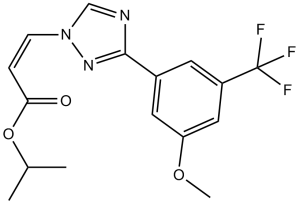
C16H16F3N3O3
|
|
|---|---|---|
| 分子量 |
355.31
|
|
| 精确质量 |
355.114
|
|
| 元素分析 |
C, 54.09; H, 4.54; F, 16.04; N, 11.83; O, 13.51
|
|
| CAS号 |
1333151-73-7
|
|
| 相关CAS号 |
|
|
| PubChem CID |
53495165
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
458.8±55.0 °C at 760 mmHg
|
|
| 闪点 |
231.3±31.5 °C
|
|
| 蒸汽压 |
0.0±1.1 mmHg at 25°C
|
|
| 折射率 |
1.526
|
|
| LogP |
4.24
|
|
| tPSA |
66.24
|
|
| 氢键供体(HBD)数目 |
0
|
|
| 氢键受体(HBA)数目 |
8
|
|
| 可旋转键数目(RBC) |
6
|
|
| 重原子数目 |
25
|
|
| 分子复杂度/Complexity |
485
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
CC(C)OC(=O)/C=C\N1C=NC(=N1)C2=CC(=CC(=C2)OC)C(F)(F)F
|
|
| InChi Key |
NLNGWFLRRRYNIL-PLNGDYQASA-N
|
|
| InChi Code |
InChI=1S/C16H16F3N3O3/c1-10(2)25-14(23)4-5-22-9-20-15(21-22)11-6-12(16(17,18)19)8-13(7-11)24-3/h4-10H,1-3H3/b5-4-
|
|
| 化学名 |
propan-2-yl (Z)-3-[3-[3-methoxy-5-(trifluoromethyl)phenyl]-1,2,4-triazol-1-yl]prop-2-enoate
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.5 mg/mL (7.04 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (7.04 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (7.04 mM) (饱和度未知) in 10% EtOH + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8144 mL | 14.0722 mL | 28.1444 mL | |
| 5 mM | 0.5629 mL | 2.8144 mL | 5.6289 mL | |
| 10 mM | 0.2814 mL | 1.4072 mL | 2.8144 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|---|
|