| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
VEGFR1 (IC50 = 22 nM); VEGFR2 (IC50 = 4 nM); VEGFR3 (IC50 = 5.2 nM); FGFR1 (IC50 = 46 nM); PDGFRα (IC50 = 51 nM); PDGFRβ (IC50 = 39 nM); c-Kit (IC50 = 100 nM); FGFR2; FGFR3; FGFR4; RET
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:E7080作为体外血管生成的有效抑制剂,对VEGF/KDR和SCF/Kit信号传导显示出显着的抑制作用。根据体外受体酪氨酸和丝氨酸/苏氨酸激酶测定,E7080 抑制 Flt-1、KDR、Flt-4,IC50 分别为 22、4.0 和 5.2 nM。除了这些激酶外,E7080 还抑制 FGFR1 和 PDGFR 酪氨酸激酶,对 FGFR1、PDGFRα 和 PDGFRβ 的 IC50 值分别为 46、51 和 100 nM。 E7080 有效抑制分别受 VEGF 和 VEGF-C 刺激的 HUVEC 中 VEGFR2(IC50,0.83 nM)和 VEGFR3(IC50,0.36 nM)的磷酸化。最近的一项研究表明,E7080 处理(1 μM 和 10 μM)可通过抑制 FGFR 和 PDGFR 信号传导显着抑制细胞迁移和侵袭。激酶测定:酪氨酸激酶测定通过 HTRF(KDR、VEGFR1、FGFR1、c-Met、EGFR)和 ELISA (PDGFRβ) 使用受体的重组激酶结构域进行。在这两种测定中,将 4 μL E7080 的连续稀释液与 10 μL 酶、16 μL 聚 (GT) 溶液 (250 ng) 和 10 μL ATP 溶液 (1 μM ATP) 混合在 96 孔圆板中( DMSO 的最终浓度为 0.1%)。在空白孔中,不添加酶。在对照孔中不添加测试物品。通过向每个孔中添加 ATP 溶液来启动激酶反应。 30°C 孵育 30 分钟后,通过向每孔的反应混合物中添加 0.5 M EDTA(10 μL/孔)来终止反应。将适合每种激酶测定的稀释缓冲液添加到反应混合物中。在 HTRF 测定中,将 50 μL 反应混合物转移至 96 孔 1/2 面积黑色 EIA/RIA 板,将 HTRF 溶液(50 μL/孔)添加到反应混合物中,然后通过以下方法测定激酶活性:使用时间分辨荧光检测器在 337 nm 激发波长和 620 和 665 nm 发射波长下测量荧光。在 ELISA 中,将 50 μL 反应混合物在亲和素包被的 96 孔聚苯乙烯板中室温孵育 30 分钟。用洗涤缓冲液洗涤后,加入PY20-HRP溶液(70μL/孔),并将反应混合物在室温下孵育30分钟。用洗涤缓冲液洗涤后,将TMB试剂(100μL/孔)添加到每个孔中。几分钟(10-30 分钟)后,向每个孔中添加 1 M H3PO4(100 μL/孔)。通过使用酶标仪测量 450 nm 处的吸光度来确定激酶活性。细胞测定:将 HUVEC(每孔 1,000 个细胞,在含有 2% 胎牛血清的无血清培养基中)和 L6 大鼠骨骼肌成肌细胞(每孔 5,000 个细胞,在无血清 DMEM 中)分配到 96 孔板中并孵育过夜。将 E7080 和含有 2% 胎牛血清的 VEGF (20 ng/mL) 或 FGF-2 (20 ng/mL) 以及 PDGFβ (40 ng/mL) 添加到每个孔中。将细胞孵育3天,然后用WST-1试剂测量存活细胞的比例。对于增殖测定,复制样品并进行三个单独的实验。
E7080的激酶抑制谱。[4] E7080的激酶抑制谱通过无细胞激酶试验确定(表1)。E7080有效抑制VEGF-R3激酶活性(IC50, 5.2 nmol/L;表1;补充图S1)和VEGF-R2激酶活性(IC50, 4.0 nmol/L)在相似程度上(表1)。E7080也抑制VEGF-R1, FGF-R1和PDGF-Rβ激酶,但抑制活性约为4至10倍(表1)。E7080不能有效抑制EGFR激酶。E7080分别对VEGF和VEGF- c刺激后HUVECs中VEGF- r2 (IC50, 0.83 nmol/L)和VEGF- r3 (IC50, 0.36 nmol/L)的磷酸化有很强的抑制作用(表1;图1)。这些数据表明E7080是VEGF-R3激酶和VEGF-R2激酶的有效抑制剂。E7080对vegf诱导的HUVEC增殖的抑制活性(IC50, 2.7 nmol/L)强于碱性FGF诱导的HUVEC增殖(IC50, 410 nmol/L)和pdgf诱导的L细胞增殖(IC50, 340 nmol/L;表1).我们无法确定VEGF-C诱导细胞增殖的IC50值,因为在我们的实验中VEGF-C没有刺激细胞增殖。 E7080抑制人乳腺癌细胞诱导的血管生成和淋巴管生成。[4] MDA-MB-231细胞是一种来源于胸腔积液的人乳腺腺癌细胞(25)。接种到mfp中的MDA-MB-231细胞转移在区域淋巴结和远端肺中发生的频率很高(表2),而MDA-MB-435细胞仅在远端肺中发生转移(数据未显示)。条件培养基的ELISA检测表明,两种肿瘤细胞都表达了大量的VEGF,但只有MDA-MB-231产生了大量的VEGF- c(表3),两种细胞系都没有产生可检测到的VEGF- d。这些数据表明,在MDA-MB-231 m.f.p.异种移植模型中,VEGF/VEGF- r2和VEGF- c /VEGF- r3信号可能被激活,导致肿瘤转移到局部淋巴结和远处肺,而在MDA-MB-435 m.f.p.异种移植模型中,只有VEGF/VEGF- r2信号可能被激活,导致肿瘤转移到远处肺。为了确定VEGF/VEGF- r2和VEGF- c /VEGF- r3信号在转移中的作用,我们检测了抗VEGF抗体贝伐单抗(VEGF信号的选择性抑制剂)和E7080 (VEGF- r2和VEGF- r3激酶的双重抑制剂)在两种m.f.p.异种移植模型中对血管生成和淋巴管生成的影响。用抗cd31抗体和抗lyve -1抗体分别对肿瘤组织进行染色,评价血管生成和淋巴管生成的程度。 |
| 体内研究 (In Vivo) |
当在 H146 异种移植模型中口服给药时,E7080 在 30 和 100 mg/kg 剂量下以剂量依赖性方式抑制 H146 肿瘤的生长,并在 100 mg/kg 剂量下导致肿瘤消退。此外,100 mg/kg 的 E7080 比抗 VEGF 抗体和伊马替尼治疗更能降低微血管密度。 E7080 显着抑制 MDA-MB-231 乳腺脂肪垫 (mfp) 模型中的局部肿瘤生长,RTV(第 8 天计算的肿瘤体积/第 1 天的肿瘤体积)为 0.81,并减少已建立的转移结节的血管生成和淋巴管生成。淋巴结中的 MDA-MB-231 肿瘤。
E7080、伊马替尼和VEGF中和抗体对H146异种移植物模型的疗效[4] 为了研究SCF/KIT信号在肿瘤血管生成中的作用,研究人员利用H146异种移植物模型,评估了同时抑制KDR和KIT激酶的E7080、选择性抑制VEGF信号的VEGF中和抗体和单独抑制KIT激酶的伊马替尼的作用。口服E7080在剂量为30和100 mg/kg (BID, QDx21)时抑制H146肿瘤生长,呈剂量依赖性,在剂量为100 mg/kg时引起肿瘤消退(图6a)。伊马替尼剂量为160 mg/kg (BID, QDx21)或抗vegf抗体剂量为300和500 μg /只小鼠(每周两次)均可明显减缓肿瘤生长,但未引起肿瘤消退(图6a)。抗cd31抗体的免疫组化分析(图6b)显示,E7080在100 mg/kg时比抗vegf抗体和伊马替尼治疗更能降低微血管密度(图6c)。E7080可能通过抑制KIT和VEGF受体信号传导而具有强大的抗血管生成活性,从而实现肿瘤消退。 在MDA-MB-231 m.f.p.异种移植模型中,E7080抑制区域淋巴结和远端肺转移。[4] 接下来,研究人员评估了E7080和贝伐单抗对MDA-MB-231转移到区域淋巴结和远端肺的影响。MDA-MB-231发生转移的时间为~ 7周。我们在接种43天后用抑制剂治疗荷瘤小鼠,并给药56天(图4)。E7080和贝伐单抗在m.f.p均显著抑制局部肿瘤生长,治疗结束时,RTVs分别为0.81±1.00 (E7080)、5.11±6.54(贝伐单抗)和17.4±13.1(载药组);P < 0.05;图4). E7080还能显著抑制肿瘤向区域淋巴结和远端肺转移(P < 0.05;表2)。E7080治疗后,10只小鼠中有0只发生淋巴结转移,10只小鼠中有0只发生肺转移,而12只小鼠中有9只发生淋巴结和肺转移。贝伐单抗似乎也降低了转移到淋巴结(10例中有6例)和肺(10例中有3例)的发生率,但这种降低仅在肺中显著(表2)。这些结果表明贝伐单抗不能抑制VEGF-C/VEGF-R3信号。 E7080降低MDA-MB-231肿瘤淋巴结转移结节的血管生成和淋巴管生成。[4] 研究人员观察到,E7080治疗原发性MDA-MB-231肿瘤的淋巴管生成和血管生成均显著减少(图3)。因此,我们评估了E7080在切除原发肿瘤后,对转移结节生长、血管生成和淋巴结转移结节内淋巴管生成的影响(图5A)。在接种后90天切除原发肿瘤(图5A),并在肿瘤切除后2周开始给予E7080,持续4周(图5C)。E7080似乎能抑制转移性结节的生长(对照:11.8±10.8;E7080: 0.6±0.3;图5B和C),但由于rtv在载药组中变化较大,因此没有统计学差异,尽管抗cd31和抗lyve -1抗体的免疫组织化学分析(图6)表明E7080处理显著降低了MVD(载药组:94.3±12.6;E7080: 20.3±2.9/mm2;图6A和C)和LVD(载体:24.7±13.3;E7080: 1.0±0.9/mm2;图6B和C)在淋巴结转移结节内。这些结果表明,E7080抑制MDA-MB-231异种移植瘤模型中淋巴结转移结节内的血管生成和淋巴管生成。 |
| 酶活实验 |
受体的重组激酶结构域用于 HTRF(KDR、VEGFR1、FGFR1、c-Met、EGFR)和 ELISA (PDGFRβ) 酪氨酸激酶测定。在这两种测定中,将 10 μL 酶、16 μL 聚 (GT) 溶液 (250 ng) 和 10 μL ATP 溶液 (1 μM ATP) 与 4 μL Lenvatinib (E7080)连续稀释液在 96 孔圆板中混合(DMSO终浓度为0.1%)。空白孔中不添加酶。对照孔中没有添加测试物品。每孔添加 ATP 溶液以启动激酶反应。在 30°C 孵育 30 分钟后,向每个孔中的反应混合物中添加 0.5 M EDTA(10 μL/孔)来终止反应。反应混合物中添加了适合每种激酶测定的稀释缓冲液。 HTRF 测定包括将 50 μL 反应混合物转移至 96 孔 1/2 面积黑色 EIA/RIA 板,每孔添加 50 μL HTRF 溶液,并使用时间分辨荧光检测器测量反应混合物的荧光发射波长为 620 和 665 nm,激发波长为 337 nm。这允许测定激酶活性。对于 ELISA,将涂有抗生物素蛋白的 96 孔聚苯乙烯板与 50 μL 反应混合物在室温下孵育 30 分钟。用洗涤缓冲液洗涤后,将反应混合物在室温下孵育 30 分钟,然后添加 PY20-HRP 溶液(70 μL/孔)。用洗涤缓冲液洗涤后,在每个孔中添加 100 μL TMB 试剂。几分钟(10-30 分钟)后,每个孔接收 100 μL 1 M H3PO4。通过使用酶标仪测量 450 nm 处的吸光度,可以鉴定激酶活性。
体外激酶测定[3] 酪氨酸激酶检测采用HTRF (KDR, VEGFR1, FGFR1, c-Met, EGFR)和ELISA (PDGFRβ),使用受体的重组激酶结构域。在两个实验中,将4 μL Lenvatinib (E7080)系列稀释剂与10 μL酶、16 μL聚(GT)溶液(250 ng)和10 μL ATP溶液(1 μmol/L ATP)(终浓度DMSO为0.1%)混合在96孔圆板中。在空白的孔中,没有添加酶。在对照井中不添加试验品。激酶反应是通过在每个孔中加入ATP溶液来启动的。30℃孵育30 min后,每孔加入0.5 mol/L EDTA (10 μL/孔)停止反应。在反应混合物中加入适合每个激酶测定的稀释缓冲液。 在HTRF实验中,将反应混合物的50 μL转移到96孔1/2面积的黑色EIA/RIA板上,在反应混合物中加入50 μL/孔的HTRF溶液,用时间分辨荧光检测器在激发波长为337 nm,发射波长为620和665 nm处测量荧光,测定激酶活性。 ELISA中,将反应液取50 μL于亲和素包被的96孔聚苯乙烯板中室温孵育30 min,用洗涤缓冲液洗涤后,加入PY20-HRP溶液(70 μL/孔),室温孵育30 min,用洗涤缓冲液洗涤后,每孔加入TMB试剂(100 μL/孔)。10 ~ 30min后,每孔加入1 mol/L H3PO4 (100 μL/孔)。激酶活性用酶标仪测定450 nm处吸光度。 ProQinase公司检测了除KDR、VEGFR1、FGFR1、c-Met、EGFR和PDGFRβ外,Lenvatinib (E7080)的激酶抑制活性。 无细胞激酶试验/细胞磷酸化试验。[4] 酪氨酸激酶活性通过均匀时间分辨荧光法(VEGF-R2、VEGF-R1、成纤维细胞生长因子受体1 (FGF-R1)和表皮生长因子受体)和ELISA法(血小板衍生生长因子(PDGF)受体β)测定,利用这些受体的重组激酶结构域。使用ProQinase公司的技术平台检测Lenvatinib (E7080)对VEGF-R3的激酶抑制活性。对于无细胞激酶试验,重复样品并进行两到三个单独的实验。HUVECs在含0.5%胎牛血清的无血清培养基中培养24小时,细胞用Lenvatinib (E7080)处理,用VEGF (20 ng/mL)或VEGF- c (100 ng/mL)刺激10分钟,然后收集在裂解缓冲液中。为了检测VEGF-R2和磷酸化的VEGF-R2,电泳10 ~ 20 μg的细胞裂解物。为了检测VEGF-R3和磷酸化的VEGF-R3,用抗VEGF-R3免疫沉淀400 ~ 1000 μg的细胞裂解物。免疫复合物在60 μL样品缓冲液中溶解,电泳。通过Western blot分析分离的蛋白与指定抗体:VEGF-R2和磷酸化VEGF-R2, VEGF-R3和抗磷酸酪氨酸IgG。使用Image Master VDS-CL化学发光显示免疫反应条带。使用1D图像分析软件测量每个波段的强度。在细胞磷酸化实验中,我们分别做了三个独立的实验。 |
| 细胞实验 |
H146(1.2×103 细胞/50 μL/孔)在 96 孔多孔板中用含有 0.5% BSA 的 SFM 培养。在 37°C 培养过夜后,添加含有 0.5% FBS 和各种 SCF 浓度的 SFM(150 μL/孔),可添加或不添加各种化合物浓度。 WST-1 用于测量 72 小时培养后存活细胞的比例。
流式细胞术分析[3] 先用胰蛋白酶分离细胞,离心后用PBS或1 μg的一抗(抗kit抗体)在4°C下孵育30分钟,然后用50 μL的抗pe偶联二抗在PBS中稀释1:50孵育。用流式细胞术分析染色细胞,用FACS Calibur仪器量化染色强度,结果显示为直方图。 增殖试验[3] 将含0.5% BSA的SFM中的H146 (1.2 × 103个细胞/50 μL/孔)培养于96孔多孔板中。37°C培养过夜后,加入含有0.5% FBS和几种SCF浓度的SFM (150 μL/孔),添加或不添加几种浓度的化合物。培养72h后,用WST-1法测定存活细胞比例。 生长因子刺激增殖试验。将HUVECs(每孔1000个细胞在含2%胎牛血清的无血清培养基中)和L6大鼠骨骼肌成肌细胞(每孔5000个细胞在无血清的DMEM中)分配在96孔板中孵育过夜。每孔加入Lenvatinib (E7080)和VEGF (20 ng/mL)或FGF-2 (20 ng/mL),其中含有2%胎牛血清和PDGFβ (40 ng/mL)。细胞孵育3 d,用WST-1试剂测定细胞存活率。增殖实验,重复样品,分别做3次实验。 |
| 动物实验 |
Clean-room conditions are used to maintain 8–12 week old, 20–25 g female BALB/c nude mice. Mice's flanks are subcutaneously (s.c.) implanted with 6.5×106 H146 tumor cells. Day 1 of the experiment occurs twelve days after the injection when mice are randomized into treatment (n = 6 or n = 5) and control (n = 12) groups. From day one to day twenty-one, lenvatinib, STI571, and VEGF neutralization antibody are given orally twice daily for lenvatinib and STI571 and twice weekly for the antibody. These substances are suspended in 0.5% methylcellulose and saline, respectively. On the designated days, tumor volume is measured and computed. Relative tumor volume (RTV) is a measure of antitumor activity that is calculated as the volume of the tumor on day 1 divided by the tumor volume at indicated days.
Tumor xenograft model [3] Female BALB/c nude mice (8–12 weeks old, 20–25 g), obtained from Charles River (Kanagawa, Japan), were used. Animals were maintained under clean-room conditions. H146 tumor cells (6.5 × 106) were implanted subcutaneously (s.c.) into the flank region of mice. Twelve days after inoculation, mice were randomized into control (n = 12) and treatment (n = 6 or n = 5) groups and this point in time was identified as day 1. Lenvatinib (E7080) and Imatinib, and VEGF neutralization antibody were suspended in 0.5% methylcellulose and saline, respectively, and administered orally twice a day for Lenvatinib (E7080) and Imatinib and twice a week for antibody from day 1 to day 21. Tumor volume was measured on the indicated days and calculated according to the following equation: tumor volume (mm3) = length × (width)2/2. Antitumor activity was shown as a relative tumor volume (RTV = calculated tumor volume at indicated days/volume on day 1). Immunohistochemical analysis of angiogenesis and lymphangiogenesis in m.f.p. xenograft models. [4] MDA-MB-231 and MDA-MB-435 tumors were removed from mice treated with either Lenvatinib (E7080) (n = 5) or bevacizumab (n = 5) for 1 wk (day 8) and without treatment (n = 5), embedded in OCT compound, frozen on dry ice, and double stained for an endothelial cell marker CD31 (with rat monoclonal anti-mouse CD31, clone MEC13.3) and a lymph endothelial cell marker (with rabbit polyclonal anti-LYVE-1). CD31 and LYVE-1 were visualized by staining with fuchsin and 3,3′-diaminobenzidine, respectively. Microvessel density (MVD) and lymphatic vessel density (LVD) were assessed by counting tumor microvessel and lymph vessel elements (four to five fields per tumor) and calculating tumor microvessel or lymph vessel densities (i.e., number of vessel elements per field). Experiments were duplicated and statistical analysis was done using the Dunnett-type multiple comparison method. Effect of Lenvatinib (E7080) on the primary tumor growth in the m.f.p. and metastases. [4] MDA-MB-231 cells highly expressing rsGFP were implanted s.c. into the flanks of nude mice. Tumor fragments (17 ± 2 mg) were prepared from 100 to 200 mm3 tumors grown s.c. and then inoculated into the m.f.p. About 2 wk after inoculation, mice were randomized into control (n = 12) and treatment groups (n = 10) at day 1. Either Lenvatinib (E7080) (in water) or bevacizumab (in saline) was administered orally once a day or i.v. twice a week, respectively, from day 1 to day 56. Antitumor activity was shown as a relative tumor volume (RTV = calculated tumor volume/day 1 tumor volume). Tumors expressing rsGFP in the lymph node and lung were detected by a fluorescence imaging detection system after 56 d of treatment. Data include the average with SD for RTV and the ratio of the number of mice bearing metastatic nodules. Experiments were duplicated and statistical analysis was conducted using the Dunnett-type multiple comparison method. Effect of Lenvatinib (E7080) on tumor growth of metastatic nodules in the lymph nodes after resection of the primary tumor. [4] rsGFP MDA-MB-231 tumor pieces were transplanted and allowed to grow until metastases were noted in the lymph nodes (∼90 d), which were detected by a fluorescence imaging detection system, and then the primary tumors were removed. Eight mice were divided into two groups. Administration of Lenvatinib (E7080) was started 2 wk after resection of the primary tumors (day 1). Lenvatinib (E7080) was administered orally once a day from day 1 to day 28. Statistical analysis was conducted using the Dunnett-type multiple comparison method. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Time to peak plasma concentration occurred from 1 to 4 hours postdose. Administration with food did not affect the extent of absorption, but decreased the rate of absorption and delayed the median Tmax from 2 hours to 4 hours. Following administration of a radiolabeled dose, approximately 64% and 25% of the radiolabel were eliminated in the feces and urine, respectively. Metabolism / Metabolites Lenvatinib is metabolized by CYP3A and aldehyde oxidase. Biological Half-Life The terminal elimination halflife of lenvatinib is approximately 28 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In large clinical trials of lenvatinib, elevations in serum aminotransferase levels were common, occurring in 52% of patients. Values greater than 5 times the upper limit of normal (ULN), however, occurred in only 3% to 5% of recipients. Serum alkaline phosphatase elevations were also common occurring in 28% of patients and were above 3 times ULN in 2%. In addition, fatal hepatic failure was reported in 3 of 1160 patients treated in preregistration clinical trials and another patient developed symptomatic but self-limited acute hepatitis with jaundice. The degree of relatedness of these events to lenvatinib therapy, however, was not defined. In the product label for lenvatinib, serum ALT, AST and alkaline phosphatase elevations are listed as adverse reactions, and acute hepatitis is mentioned as a rare occurrence. Monitoring of serum enzymes before, every 2 weeks for 2 months and monthly thereafter during treatment is recommended with dose reduction or discontinuation depending upon the degree and persistence of the abnormalities. Likelihood score: D (possible cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of lenvatinib during breastfeeding. Because lenvatinib is more than 98% bound to plasma proteins, the amount in milk is likely to be low. However, its half-life is about 28 hours and it might accumulate in the infant. The manufacturer recommends that breastfeeding be discontinued during lenvatinib therapy and for at least 1 week after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding In vitro binding of lenvatinib to human plasma proteins ranged from 98% to 99%. |
| 参考文献 |
|
| 其他信息 |
Pharmacodynamics
Based on x-ray crystallography and kinetic interaction studies, lenvatinib binds to the adenosine 5'-triphosphate binding site of VEGFR2 and to a neighbouring region via a cyclopropane ring and thereby inhibits tyrosine kinase activity and associated signalling pathways. |
| 分子式 |
C21H19CLN4O4
|
|---|---|
| 分子量 |
426.85
|
| 精确质量 |
426.109
|
| 元素分析 |
C, 59.09; H, 4.49; Cl, 8.30; N, 13.13; O, 14.99
|
| CAS号 |
417716-92-8
|
| 相关CAS号 |
Lenvatinib mesylate;857890-39-2;Lenvatinib-d4;Lenvatinib-d5
|
| PubChem CID |
9823820
|
| 外观&性状 |
Off-white to light yellow solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 沸点 |
627.2±55.0 °C at 760 mmHg
|
| 闪点 |
333.1±31.5 °C
|
| 蒸汽压 |
0.0±1.8 mmHg at 25°C
|
| 折射率 |
1.697
|
| LogP |
3.39
|
| tPSA |
115.57
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
30
|
| 分子复杂度/Complexity |
634
|
| 定义原子立体中心数目 |
0
|
| SMILES |
ClC1C([H])=C(C([H])=C([H])C=1N([H])C(N([H])C1([H])C([H])([H])C1([H])[H])=O)OC1C([H])=C([H])N=C2C([H])=C(C(C(N([H])[H])=O)=C([H])C=12)OC([H])([H])[H]
|
| InChi Key |
WOSKHXYHFSIKNG-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C21H19ClN4O4/c1-29-19-10-17-13(9-14(19)20(23)27)18(6-7-24-17)30-12-4-5-16(15(22)8-12)26-21(28)25-11-2-3-11/h4-11H,2-3H2,1H3,(H2,23,27)(H2,25,26,28)
|
| 化学名 |
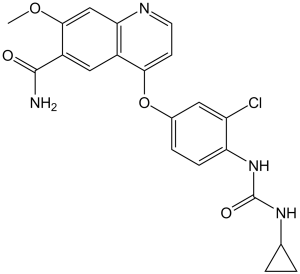
4-[3-chloro-4-(cyclopropylcarbamoylamino)phenoxy]-7-methoxyquinoline-6-carboxamide
|
| 别名 |
E-7080; E7080; E 7080; ER-203492-00; Lenvatinib; Brand name: Lenvima
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 0.64 mg/mL (1.50 mM) (饱和度未知) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
*20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: 0.5% methylcellulose: 30 mg/kg View More
配方 3 中的溶解度: 6.67 mg/mL (15.63 mM) in 0.5% Methylcellulose/saline water (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3427 mL | 11.7137 mL | 23.4274 mL | |
| 5 mM | 0.4685 mL | 2.3427 mL | 4.6855 mL | |
| 10 mM | 0.2343 mL | 1.1714 mL | 2.3427 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Safety and Efficacy Study of Pembrolizumab (MK-3475) Combined With Lenvatinib (MK-7902/E7080) as First-line Intervention in Adults With Advance Melanoma (MK-7902-003/E7080-G000-312/LEAP-003)
CTID: NCT03820986
Phase: Phase 3 Status: Completed
Date: 2024-12-02
|
|
|
|