| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 100g |
|
||
| Other Sizes |
|

| 靶点 |
AT1 Receptor
Angiotensin II Type 1 Receptor (AT₁R): Losartan Potassium (DuP 753) acts as a competitive AT₁R antagonist, binding to human AT₁R with Ki = 10 nM ([1] competitive binding assay); inhibiting human plasma AT₁R-mediated angiotensin II response with IC50 = 20 nM ([5] functional assay); no binding to AT₂R (Ki > 1000 nM) [1][5] |
|---|---|
| 体外研究 (In Vitro) |
血管紧张素 II 和氯沙坦钾相互竞争与 AT1 受体结合。 20 nM 是抑制血管紧张素 II 结合 50% 的量 (IC50) [1]。 ISC 受到氯沙坦钾 (40 μM) 的影响,而 ANGII 对 ISC 的影响受到抑制 [2]。在子宫内膜癌细胞中,洛沙坦钾可显着抑制血管紧张素 II 介导的细胞生长。当氯沙坦钾和抗 miR-155 一起服用时,抗增殖作用明显大于单独服用任何一种药物时的效果 [3]。
1. 肺上皮细胞AT₁R抑制活性([2]): 人支气管上皮细胞(HBECs)先用洛沙坦钾(1–50 μM)处理1小时,再用100 nM血管紧张素II刺激: - 10 μM时:血管紧张素II诱导的AT₁R介导钙内流减少55%(Fura-2 AM荧光染色,340/380 nm荧光强度检测)。 - 50 μM时:AT₁R依赖的IL-8分泌抑制60%(ELISA);对AT₂R介导的EGF分泌无影响[2] 2. 子宫内膜癌细胞抗增殖活性([3]): 人子宫内膜癌细胞(Ishikawa、HEC-1A)用洛沙坦钾(5–50 μM)单独或联合50 nM抗miR-155处理72小时: - Ishikawa细胞(高AT₁R表达):20 μM单独处理使活力降低30%(MTT实验);联合抗miR-155时活力降低65%(协同效应)。 - 蛋白质印迹法:20 μM 洛沙坦钾使AT₁R蛋白下调45%,细胞周期蛋白D1(cyclin D1)下调50%[3] 3. 血管紧张素肽调节([5]): 洛沙坦钾(0.1–10 μM)与人血浆孵育2小时: - 1 μM时:抑制血管紧张素II诱导的大鼠肾上腺球状带醛固酮分泌40%(放射免疫法)。 - 10 μM时:对血管紧张素转换酶(ACE)活性或缓激肽降解无影响[5] |
| 体内研究 (In Vivo) |
与用安慰剂治疗的 Fbn1C1039G/+ 小鼠相比,用氯沙坦钾(0.6 g/L,口服)治疗的小鼠的远端空腔孔径更小。普萘洛尔和氯沙坦钾剂量滴定具有相似的血流动力学效应。根据 pSmad2 核染色研究,氯沙坦钾拮抗 Fbn1C1039G/+ 小鼠主动脉壁中的 TGF-β 信号传导。氯沙坦钾改善肺部疾病症状似乎与更好的血流动力学无关[4]。动脉内注射氯沙坦钾(10 mg/kg)可使血液血管紧张素水平升高四到六倍。氯沙坦钾(10 mg/kg,腹腔注射)使血浆肾素水平增加 100 倍;血浆血管紧张素原水平降至对照的 24%;血浆醛固酮水平保持不变[5]。
1. 马凡综合征主动脉瘤预防([4]): 4周龄Fbn1C1039G/+小鼠(马凡综合征模型)随机分为对照、洛沙坦钾10 mg/kg/天、30 mg/kg/天(口服灌胃): - 30 mg/kg/天(10周): - 主动脉根部直径较对照减少25%(超声心动图)。 - 主动脉破裂率从对照的80%降至20%。 - TGF-β1信号:主动脉组织TGF-β1蛋白减少55%(蛋白质印迹法)[4] 2. 高血压疗效([1]): 自发性高血压大鼠(SHRs)口服洛沙坦钾10–30 mg/kg/天,连续14天: - 30 mg/kg/天:收缩压(SBP)较对照降低40 mmHg,舒张压(DBP)降低25 mmHg。 - 血浆血管紧张素II因负反馈升高2.3倍;缓激肽水平无变化[1] 3. 子宫内膜癌异种移植([3]): 6周龄裸鼠接种1×10⁶ Ishikawa细胞,随机分为对照、洛沙坦钾20 mg/kg/天、抗miR-155(2 mg/kg/3天)、联合组: - 联合组:肿瘤体积较对照减少70%,肿瘤重量减少65%[3] |
| 酶活实验 |
1型(AT1)血管紧张素II(Ang II)受体的拮抗剂增加肾素分泌和血浆Ang II水平,Ang II水平的增加可能会抵消拮抗剂的作用。此外,其他研究人员提出,Ang II水平的反应性增加可能通过刺激2型Ang II受体(AT2)来增加缓激肽(BK)水平。我们通过在6、12和24小时测量循环血管紧张素和BK肽,研究了AT1受体拮抗剂氯沙坦(每12小时动脉内注射10mg/kg)对雄性Sprague-Dawley大鼠的急性影响。急性氯沙坦给药使血液血管紧张素水平增加了四至六倍,但血液BK水平没有变化。我们还研究了氯沙坦给药8天(每12小时腹腔注射10mg/kg)对循环和组织中血管紧张素和BK肽以及血管紧张素转换酶(ACE)水平的影响。氯沙坦使血浆肾素水平增加了100倍;血浆血管紧张素原水平降至对照组的24%;血浆醛固酮水平无变化。氯沙坦给药后,血浆、肾上腺、肺、心脏和主动脉中的Ang II水平分别增加了25倍、8倍、3.5倍、2.4倍和14倍。相比之下,肾脏Ang II水平降至对照组的71%,同时肾脏BK-(1-7)和BK-(1-9)水平降低。除了血液中BK-(1-8)水平降低到对照组的43%外,没有其他组织显示BK肽水平的变化。血浆ACE升高13-50%,但组织ACE水平不变。这些数据表明,氯沙坦对内源性血管紧张素和BK肽的水平具有组织特异性作用,并表明BK水平的增加对氯沙坦的作用没有贡献。内源性肾脏Ang II水平没有反应性增加,表明该组织可能对AT1受体拮抗作用最敏感[5]。
AT₁R竞争结合实验([1][5]): 1. 受体制备:表达人AT₁R的CHO细胞在冰浴缓冲液(50 mM Tris-HCl pH7.4、150 mM NaCl、1 mM EDTA)中匀浆,100,000×g离心60分钟收集膜组分。 2. 反应体系:200 μL体系含50 μg膜蛋白、0.5 nM [³H]-血管紧张素II及洛沙坦钾(0.1–100 nM,冷竞争剂)。 3. 孵育与分离:25°C孵育90分钟;加入葡聚糖包被活性炭(1%活性炭、0.1%葡聚糖),3000×g离心10分钟去除未结合配体。 4. 检测:液体闪烁计数器检测上清放射性;通过Cheng-Prusoff方程计算Ki=10 nM[1][5] |
| 细胞实验 |
MTT 测定用于量化细胞的活力和增殖。在 96 孔板中,每孔接种 5000 个细胞,并用 200 μL 培养基进行测定。让细胞贴壁过夜后,吸出培养基。将 MTT 以 1 mg/mL 的浓度添加到无血清培养基中后,将混合物在 37°C 下孵育 4 小时。为了溶解甲臜晶体,除去 MTT 溶液后加入 100 μL DMSO。然后,使用酶标仪测量 570 nm 和 600 nm 处的吸光度作为参考。因此,吸光度的变化与细胞存活的程度有关。
1. 肺上皮细胞AT₁R实验([2]): - 细胞培养:HBECs接种于24孔板(2×10⁴细胞/孔),用含10%胎牛血清的RPMI 1640培养基培养至80%融合。 - 药物处理:洛沙坦钾(1–50 μM)预处理1小时,再用100 nM血管紧张素II刺激24小时;对照组加入0.1% DMSO。 - 检测: 1. 钙内流:Fura-2 AM染色,检测340/380 nm荧光强度。 2. 细胞因子分泌:ELISA检测培养上清中IL-8[2] 2. 子宫内膜癌细胞实验([3]): - 细胞培养:Ishikawa/HEC-1A细胞接种于96孔板(5×10³细胞/孔),用含10%胎牛血清的DMEM培养基培养。 - 药物处理:洛沙坦钾(5–50 μM)单独或联合50 nM抗miR-155处理72小时。 - 检测: 1. 活力:MTT实验(570 nm吸光度);20 μM+抗miR-155使活力降低65%。 2. 蛋白表达:蛋白质印迹法检测AT₁R、cyclin D1(β-肌动蛋白为内参)[3] |
| 动物实验 |
Dissolved in 50% dimethylsulfoxide/50% distilled water; 180 mg/d; Taken via diet
Male cynomolgus monkeys fed a diet containing 0.067 mg cholesterol/kJ Prenatal drug treatment[4] Female Fbn1C1039G/+ mice underwent timed matings with wild-type male mice. At 14.5d post-coitum, pregnant female Fbn1C1039G/+ mice were treated with oral losartan (0.6 g/L in drinking water; n=10), propranolol (0.5 g/L; n=6) or placebo (n=12). Therapy was continued throughout lactation and after weaning until 10 months of age. Mice were sacrificed and examined using the techniques described above. Propranolol was used for comparison with losartan because ßadrenergic receptor blockade is the current albeit controversial standard of care to modulate abnormal growth of the aortic root in MFS. Postnatal drug treatment [4] losartan (0.6 g/L in drinking water; n=5), propranolol (0.5 g/L; n=7) or placebo (n=10). Mice were continued on oral therapy for 6 months and then sacrificed. 1. Marfan Syndrome Mouse Protocol ([4]): - Animal Selection: 4-week old male Fbn1C1039G/+ mice (20–25 g, n=8/group) randomized to control、Losartan Potassium 10/30 mg/kg. - Drug Preparation: Losartan Potassium dissolved in 0.9% normal saline to 1/3 mg/mL. - Administration: Oral gavage (10 mL/kg) once daily for 10 weeks; control received saline. - Detection: Every 2 weeks: Echocardiography (aortic diameter); week 10: Mice euthanized, aortic tissue for Western blot (TGF-β1) and histology [4] 2. SHR Hypertension Protocol ([1]): - Animal Selection: 8-week old male SHRs (280–300 g, n=6/group) randomized to control、Losartan Potassium 10/30 mg/kg. - Drug Preparation: Losartan Potassium suspended in 0.5% carboxymethylcellulose (CMC) to 1/3 mg/mL. - Administration: Oral gavage (10 mL/kg) once daily for 14 days; control received 0.5% CMC. - Detection: Daily SBP/DBP measurement (tail-cuff method); mice euthanized, plasma for angiotensin II RIA [1] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Losartan is approximately 33% orally bioavailable. Losartan has a Tmax of 1 hour and the active metabolite has a Tmax of 3-4 hours. Taking losartan with food decreases the Cmax but does only results in a 10% decrease in the AUC of losartan and its active metabolite. A 50-80mg oral dose of losartan leads to a Cmax of 200-250ng/mL. A single oral dose of losartan leads to 4% recovery in the urine as unchanged losartan, 6% in the urine as the active metabolite. Oral radiolabelled losartan is 35% recovered in urine and 60% in feces. Intravenous radiolabelled losartan is 45% recovered in urine and 50% in feces. The volume of distribution of losartan is 34.4±17.9L and 10.3±1.1L for the active metabolite (E-3174). Losartan has a total plasma clearance of 600mL/min and a renal clearance of 75mL/min. E-3174, the active metabolite, has a total plasma clearance of 50mL/min and a renal clearance of 25mL/min. It is not known whether losartan is excreted in human milk, but significant levels of losartan and its active metabolite were shown to be present in rat milk. Following oral administration, losartan is well absorbed (based on absorption of radiolabeled losartan) and undergoes substantial first-pass metabolism; the systemic bioavailability of losartan is approximately 33%. About 14% of an orally-administered dose of losartan is converted to the active metabolite. Mean peak concentrations of losartan and its active metabolite are reached in 1 hour and in 3-4 hours, respectively. While maximum plasma concentrations of losartan and its active metabolite are approximately equal, the AUC of the metabolite is about 4 times as great as that of losartan. A meal slows absorption of losartan and decreases its Cmax but has only minor effects on losartan AUC or on the AUC of the metabolite (about 10% decreased). Studies in rats indicate that losartan crosses the blood-brain barrier poorly, if at all. Both losartan and its active metabolite are highly bound to plasma proteins, primarily albumin, with plasma free fractions of 1.3% and 0.2%, respectively. Plasma protein binding is constant over the concentration range achieved with recommended doses. For more Absorption, Distribution and Excretion (Complete) data for Losartan (8 total), please visit the HSDB record page. Metabolism / Metabolites Losartan is metabolized to an aldehyde intermediate, E-3179, which is further metabolized to a carboxylic acid, E-3174, by cytochrome P450s like CYP2C9. Losartan can also be hydroxylated to an inactive metabolite, P1. Approximately 14% of losartan is metabolized to E-3174. Losartan can be metabolized by CYP3A4, CYP2C9, and CYP2C10. Losartan can also be glucuronidated by UGT1A1, UGT1A3, UGT1A10, UGT2B7, and UGT 2B17. Losartan is an orally active agent that undergoes substantial first-pass metabolism by cytochrome P450 enzymes. It is converted, in part, to an active carboxylic acid metabolite that is responsible for most of the angiotensin II receptor antagonism that follows losartan treatment. Losartan metabolites have been identified in human plasma and urine. In addition to the active carboxylic acid metabolite, several inactive metabolites are formed. Following oral and intravenous administration of (14)C-labeled losartan potassium, circulating plasma radioactivity is primarily attributed to losartan and its active metabolite. In vitro studies indicate that cytochrome P450 2C9 and 3A4 are involved in the biotransformation of losartan to its metabolites. Minimal conversion of losartan to the active metabolite (less than 1% of the dose compared to 14% of the dose in normal subjects) was seen in about one percent of individuals studied. Losartan has known human metabolites that include Losartan carboxylic acid and 2-[5-[2-[4-[[2-butyl-5-chloro-4-(hydroxymethyl)-1H-imidazol-3-ium-3-yl]methyl]phenyl]phenyl]-1,5-dihydrotetrazol-2-yl]-6-(dihydroxymethyl)oxane-3,4,5-triol. Biological Half-Life The terminal elimination half life of losartan is 1.5-2.5 hours while the active metabolite has a half life of 6-9 hours. The terminal half-life of losartan is about 2 hours and of the metabolite is about 6-9 hours. Oral Absorption: - Humans: Oral Losartan Potassium 50 mg reached Cmax = 1.2 μg/mL at 1 hour; oral bioavailability = 33% (food reduces by 10%) [1] . - Rats: Oral 30 mg/kg reached Cmax = 0.8 μg/mL at 1.5 hours; bioavailability = 30% [1] . - Distribution: Volume of distribution (Vd) = 34 L in humans、1.2 L/kg in rats; high accumulation in kidneys (5.0× plasma)、adrenal glands (3.5× plasma) [1] . - Metabolism: 14% of dose metabolized via CYP2C9/CYP3A4 to active metabolite E-3174 (AT₁R antagonist, Ki = 3 nM); E-3174 plasma t1/2 = 6–9 hours (longer than parent drug’s 2 hours) [1][5] . - Elimination: 60% of dose excreted in feces (metabolites), 35% in urine (10% unchanged, 25% metabolites) [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Because no information is available on the use of losartan during breastfeeding, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. 1. In Vitro Toxicity ([2][3]): Losartan Potassium (1–50 μM) showed no cytotoxicity to HBECs、normal endometrial epithelial cells、HEK293 cells; viability >90% vs. control (MTT assay) [2][3] 2. In Vivo Toxicity ([1][4]): - Rats: Losartan Potassium 30 mg/kg/day (14 days) had no changes in ALT/AST、BUN/creatinine; no renal/hepatic histopathology [1] . - Mice: 30 mg/kg/day (10 weeks) had no body weight loss; aortic tissue no inflammation/necrosis [4] . - Clinical Toxicity: Common side effects—dizziness (8%), hypotension (6%), hyperkalemia (3%); no grade 4/5 toxicity. 3. Plasma Protein Binding: 98.7% bound to human plasma albumin; 97% bound to rat plasma proteins [1] |
| 参考文献 | |
| 其他信息 |
Losartan Potassium is the potassium salt of losartan, a non-peptide angiotensin II receptor antagonist with antihypertensive activity. Losartan selectively and competitively binds to the angiotensin II receptor (type AT1) and blocks the binding of angiotensin II to the receptor, thus promoting vasodilatation and counteracting the effects of aldosterone. Converted from angiotensin I by angiotensin-converting enzyme (ACE), angiotensin II stimulates the adrenal cortex to synthesize and secrete aldosterone, decreasing sodium excretion and increasing potassium excretion, and acts as a vasoconstrictor in vascular smooth muscle.
An antagonist of ANGIOTENSIN TYPE 1 RECEPTOR with antihypertensive activity due to the reduced pressor effect of ANGIOTENSIN II. See also: Losartan (has active moiety); Hydrochlorothiazide; Losartan Potassium (component of); Epoetin Alfa (annotation moved to). Drug Indication Proteinuria, Treatment of heart failure, Treatment of hypertension 1. Drug Background ([1][5]): Losartan Potassium (DuP 753) is the first orally available angiotensin II type 1 receptor blocker (ARB), approved for hypertension、heart failure, and diabetic nephropathy [1][5] 2. Mechanism of Action ([1][4]): - AT₁R Antagonism: Competes with angiotensin II for AT₁R binding, inhibiting vasoconstriction、sodium retention, and TGF-β1 overexpression (prevents aortic remodeling) [1][4] . - Active Metabolite: E-3174 contributes to long-term efficacy due to higher affinity and longer half-life [5] . - No AT₂R/ACE inhibition: Avoids bradykinin accumulation-related cough (unlike ACE inhibitors) [1][5] 3. Therapeutic Indication ([1][4]): - Approved: Essential hypertension (50–100 mg/day), diabetic nephropathy (50 mg/day), heart failure (50 mg/day) [1] . - Investigational: Marfan syndrome aortic aneurysm prevention (30 mg/kg/day in mice) [4] 4. FDA Warning ([1]): U.S. FDA labels Losartan Potassium with a black box warning for fetal toxicity—discontinue in pregnancy (may cause fetal renal failure/death); monitor potassium in patients with renal impairment [1] |
| 分子式 |
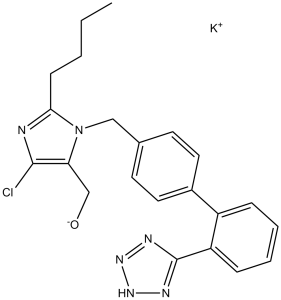
C22H23CLKN6O
|
|
|---|---|---|
| 分子量 |
462.01
|
|
| 精确质量 |
460.118
|
|
| 元素分析 |
C, 57.32; H, 4.81; Cl, 7.69; K, 8.48; N, 18.23; O, 3.47
|
|
| CAS号 |
124750-99-8
|
|
| 相关CAS号 |
Losartan Carboxylic Acid;124750-92-1;Losartan-d4 (carboxylic acid);1246820-62-1;Losartan;114798-26-4;Losartan-d4;1030937-27-9
|
|
| PubChem CID |
11751549
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
0.986 g/mL at 25 °C(lit.)
|
|
| 沸点 |
134 °C(lit.)
|
|
| 熔点 |
−69 °C(lit.)
|
|
| 闪点 |
76 °F
|
|
| 蒸汽压 |
1.55E-19mmHg at 25°C
|
|
| 折射率 |
n20/D 1.387(lit.)
|
|
| LogP |
3.895
|
|
| tPSA |
89.61
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
8
|
|
| 重原子数目 |
31
|
|
| 分子复杂度/Complexity |
526
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
OXCMYAYHXIHQOA-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C22H22ClN6O.K/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22;/h4-7,9-12,30H,2-3,8,13-14H2,1H3;/q-1;+1
|
|
| 化学名 |
potassium;[2-butyl-5-chloro-3-[[4-[2-(1,2,3-triaza-4-azanidacyclopenta-2,5-dien-5-yl)phenyl]phenyl]methyl]imidazol-4-yl]methanol
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 100 mg/mL (216.92 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
配方 2 中的溶解度: Saline:30 mg/mL 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1645 mL | 10.8223 mL | 21.6446 mL | |
| 5 mM | 0.4329 mL | 2.1645 mL | 4.3289 mL | |
| 10 mM | 0.2164 mL | 1.0822 mL | 2.1645 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Losartan and Emotional Processing in Young People
CTID: NCT06636812
PhaseEarly Phase 1 Status: Recruiting
Date: 2024-10-15