| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
Loxoprofen 是一种抗炎前药 (NSAID) 和非选择性 COX 抑制剂,在人全血测定中,COX-1 和 COX-2 的 IC50 值分别为 6.5 和 13.5 μM [1]。洛索洛芬 (LOX) 是一种非选择性环氧合酶抑制剂,常用于研究慢性和暂时性疾病的疼痛和炎症。其醇代谢物由羰基还原酶(CR)产生,由活性反式LOX和非活性顺式LOX组成。此外,LOX还可被细胞色素P450(CYP)转化为无活性的羟基化代谢物(OH-LOXs)[2]。
|
|---|---|
| 体内研究 (In Vivo) |
通过降低炎症,洛索洛芬钠(4 mg/kg/天;口服;1 或 8 周)可降低小鼠的动脉粥样硬化 [3]。通过阻断 VEGF,洛索洛芬钠(60 μg/mL;口服;24 天)可防止小鼠肿瘤生长 [4]。
|
| 动物实验 |
Animal/Disease Models: ApoE-/- mice (C57BL/6J-Apoetm1Unc), 8 to 16 weeks old, high-fat diet (0.2% cholesterol, 21% saturated fat) [3]
Doses: 4 mg/kg/day, given in drinking water Medication method: Oral administration at 8 to 16 weeks of age or 15 to 16 weeks of age. Experimental Results: Inhibition of platelet thromboxane production and platelet aggregation. Reduce the extent of atherosclerosis. Inhibits the production of PGE2, TxB2 and PGI2. Animal/Disease Models: 6weeks old male C57BL/6 and BDF1 mice, 100 μL suspension of LLC cells and KLN205 cells (2×106 cells/mL) were subcutaneously (sc) (sc) injected into C57BL/6 and BDF1 mice respectively [4]. Doses: 60 μg/mL Route of Administration: Orally administered daily for 24 days Experimental Results: Inhibited tumor growth and angiogenesis in LLC tumor mice, inhibited VEGF expression, and inhibited HUVEC tube formation. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Loxoprofen is rapidly and completely absorbed from the GI tract with a bioavailability of 95%. The absorption phase of the medication occurs in the first 4-6 hours after ingestion. Food ingestion with the medication causes a slight decrease in the rate of loxoprofen absorption. 50% renal excretion. This drug is 20% - 30% excreted in the stool. Loxoprofen has a volume of distribution of 0.16 L/kg. Most of the drug as unchanged loxoprofen, 6-0-desmethyl loxoprofen (less than 1%) and glucuronide or other conjugates (66-92%). In patients with renal failure, metabolites may accumulate. Metabolism / Metabolites Loxoprofen is a prodrug that is rapidly converted to its active trans-alcohol metabolite by carbonyl reductase in the liver. This same process also results in a cis-alcohol metabolite, though this isomer carries little pharmacological activity. The parent drug has also been observed to undergo oxidation via CYP3A4/5 to two hydroxylated metabolites (M3 and M4) and glucuronidation by UGT2B7 to two glucuronide metabolites (M5 and M6). The alcohol metabolites of loxoprofen also undergo glucuronide conjugation via UGT2B7 to two glucuronide metabolites (M7 and M8) prior to excretion. When applied in topical formulations, loxoprofen is metabolized to its active trans-alcohol form by carbonyl reductase in the skin. Biological Half-Life The elimination half-life of Loxoprofen is approximately 15 hours. Steady concentration is achieved after 2-3 doses. |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
99% albumin-bound. At doses of loxoprofen greater than 500 mg/day, clearance of the drug increases as saturation of plasma protein binding occurs at higher doses. |
| 参考文献 |
|
| 其他信息 |
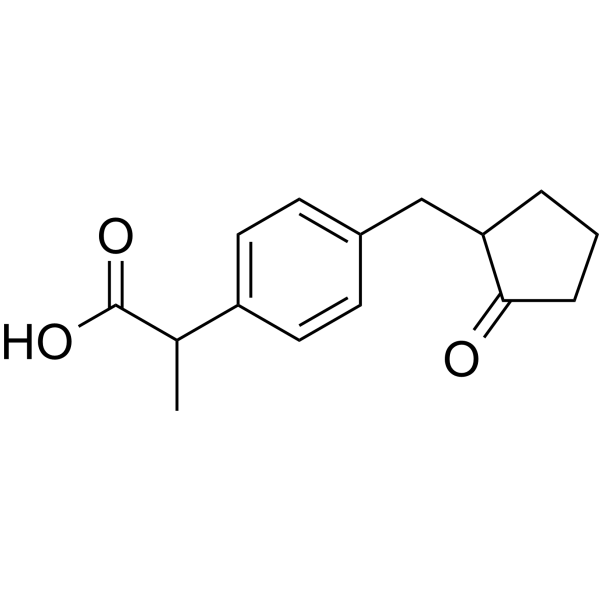
Loxoprofen is a monocarboxylic acid that is propionic acid in which one of the hydrogens at position 2 is substituted by a 4-[(2-oxocyclopentyl)methyl]phenyl group. A prodrug that is rapidly converted into its active trans-alcohol metabolite following oral administration. It has a role as a non-steroidal anti-inflammatory drug, a non-narcotic analgesic, an antipyretic, an EC 1.14.99.1 (prostaglandin-endoperoxide synthase) inhibitor and a prodrug. It is a monocarboxylic acid and a member of cyclopentanones. It is functionally related to a propionic acid. It is a conjugate acid of a loxoprofen(1-).
Loxoprofen is a propionic acid derivative non-steroidal anti-inflammatory drug. It is marketed under the trade name Loxonin in Brazil, Mexico and Japan by Sankyo, as Loxomac in India, and as Oxeno in Argentina. A transdermal preparation was approved for use in Japan in January 2006. Drug Indication Loxoprofen is non-steroidal anti-inflammatory medication (NSAID) indicated for pain and inflammation related to musculoskeletal and joint disorders. In addition to its effects on pain, it is an antipyretic and anti-inflammatory medication. Mechanism of Action Loxoprofen itself is a prodrug and carries little-to-no pharmacological activity - it is rapidly metabolized to its trans-alcohol form, which is a potent and non-selective inhibitor of cyclooxygenase. Cyclooxygenase (COX) is present in 2 forms, COX-1 and COX-2, with each serving different functions. COX-1 is present in human cells and is constitutively released, performing cellular housekeeping functions such as mucus production and platelet aggregation. COX-2 is induced in human cells post-injury or due to other stimuli, is triggered to appear in large quantities at the sites of injury/stimuli, and is ultimately responsible for the mediation of inflammation and pain. Loxoprofen's active metabolite inhibits both COX isoforms, resulting in reduced expression of several mediators of pain, inflammation, and fever (e.g. prostaglandins, prostacyclin, thromboxane, etc). Pharmacodynamics Loxoprofen is a non-selective inhibitor of cyclooxygenase enzymes, which are responsible for the formation of various biologically active pain, fever, and inflammatory mediators. These include prostaglandins, prostacyclin, thromboxane, and arachidonic acid. |
| 分子式 |
C15H18O3
|
|---|---|
| 分子量 |
246.3016
|
| 精确质量 |
246.125
|
| CAS号 |
68767-14-6
|
| 相关CAS号 |
Loxoprofen sodium;80382-23-6;Loxoprofen sodium (dihydrate);226721-96-6;Loxoprofen-d4
|
| PubChem CID |
3965
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
417.9±20.0 °C at 760 mmHg
|
| 熔点 |
108.5 - 111ºC
|
| 闪点 |
220.7±18.3 °C
|
| 蒸汽压 |
0.0±1.0 mmHg at 25°C
|
| 折射率 |
1.568
|
| LogP |
1.87
|
| tPSA |
54.37
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
18
|
| 分子复杂度/Complexity |
316
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
YMBXTVYHTMGZDW-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C15H18O3/c1-10(15(17)18)12-7-5-11(6-8-12)9-13-3-2-4-14(13)16/h5-8,10,13H,2-4,9H2,1H3,(H,17,18)
|
| 化学名 |
2-[4-[(2-oxocyclopentyl)methyl]phenyl]propanoic acid
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 100 mg/mL (~406.01 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (10.15 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (10.15 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (10.15 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.0601 mL | 20.3004 mL | 40.6009 mL | |
| 5 mM | 0.8120 mL | 4.0601 mL | 8.1202 mL | |
| 10 mM | 0.4060 mL | 2.0300 mL | 4.0601 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Effects of 2-week celecoxib treatment on the small intestinal mucosa in Japanese healthy subjects evaluated by capsule endoscopy (A prospective, randomized, double-blind, parallel-group, controlled study compared to the combination of loxoprofen sodium and lansoprazole)
CTID: UMIN000007936
Phase: Status: Complete: follow-up complete
Date: 2012-05-13