| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
|
| 靶点 |
sphingomyelin synthase (SMS)(IC50 = 1.5~3 μM)
|
|---|---|
| 体外研究 (In Vitro) |
研究了malabarine C(mal C)对人乳腺癌症MCF-7细胞系的细胞毒性机制。Mal C剂量依赖性地增加了亚G1细胞群,与细胞质寡核小体形成和染色质凝缩有关。mal C诱导的凋亡导致线粒体损伤,如JC-1染色细胞的荧光显微镜和流式细胞术所示,以及线粒体特异性核酸酶蛋白AIF和内切G的释放。mal C也从MCF-7细胞中释放细胞内Ca(2+),但Ca(2-)调节剂BAPTA-AM和Ru360仅部分消除了凋亡。malC激活钙蛋白酶对其细胞毒性没有任何影响。另一方面,在malC处理后,比线粒体损伤更早地观察到明显的溶酶体膜透性(LMP),以及组织蛋白酶B的释放、Bid切割及其向线粒体的易位。这表明mal C对人MCF-7人癌症细胞系的细胞毒性可能通过LMP作为触发胱天蛋白酶非依赖性但组织蛋白酶B和t-Bid依赖性内在线粒体凋亡途径的初始事件进行。S期或G2-M期细胞的显著积累,以及由于mal C暴露引起的细胞周期蛋白E和A的上调,使其有望成为潜在的抗癌剂。[3]
malabarine C(mal C)抑制T细胞活化、增殖和细胞因子产生。 malabarine C(mal C)抑制丝裂原诱导的细胞表面标志物MAPK和NF-κB的激活。 malabarine C(mal C)调节淋巴细胞中的细胞氧化还原状态[2]。 |
| 体内研究 (In Vivo) |
天然存在的抑制剂和靶向膜蛋白之间的相互作用可能是治疗代谢综合征,特别是肥胖的替代药物策略。在这项研究中,我们鉴定了从肉豆蔻果实中分离出的马拉巴锥A-C和E(1-4)作为鞘磷脂合酶(SMS)的天然抑制剂,SMS是一种负责鞘脂生物合成的膜蛋白。在高脂饮食诱导的肥胖小鼠模型中,口服化合物3/malabaricone C(mal C)具有最有前景的抑制作用,在减少体重增加、改善葡萄糖耐量和减少肝脂肪变性方面表现出多种功效。肝脏脂质分析揭示了化合物3的SMS活性与其体内外脂质代谢之间的关键联系。化合物3/malabaricone C(mal C)的无毒性使其成为寻找治疗和预防肥胖药物的合适候选者。[1]
malabarine C(mal C)给药抑制了离体T细胞活化[2] 为了确定malabarine C(mal C)作为体内免疫抑制剂的能力,小鼠腹腔注射载体(DMSO)或mal C,24小时后分离淋巴细胞。然后,用CFSE染色细胞并用Con A刺激。与载体对照组相比,mal C给药显著降低了增殖细胞的百分比(图4A和B)。Mal C给药导致T细胞离体分泌丝裂原诱导的细胞因子(IL-2、IFN-γ和IL-6)显著减少 为了研究malabarine C(mal C)预防急性GvHD的效用,将C57BL/6小鼠的淋巴细胞用mal C(10μM)处理4小时,并用作同种异体供体细胞。受体BALB/c小鼠通过暴露于6 Gy剂量的WBI而出现淋巴细胞减少。供体细胞通过侧尾静脉注射(每只小鼠1000万个细胞)过继转移,并监测GvHD宿主的发病率和死亡率。用载体处理的同种异体细胞重组的小鼠(GvHD组)在30天内死亡。然而,供体细胞的Mal C治疗完全抑制了GvHD相关的死亡率和发病率(图4D和E)。与未受辐射的对照组相比,GvHD相关的体重减轻在第10天很明显,这在用Mal C处理的细胞重建的小鼠中得到了显著预防(图4E)。与未受照射的对照组相比,接受WBI(6 Gy)的小鼠IL6水平显著升高(图4F)。GvHD相关IFN-γ水平显著高于WBI 6Gy组。此外,与接受载体处理的供体细胞的宿主相比,用Mal C处理的供体电池重构的宿主中IL6和IFN-γ的血清水平降低(图4F)。图4G显示了Mal C治疗对淋巴细胞减少宿主中同基因CD4+T细胞稳态增殖的影响。Mal C治疗不影响CD4+T细胞的稳态增殖[2]。 |
| 细胞实验 |
表面染色和细胞内染色[2]
如前所述,用荧光染料(PE或FITC)偶联的单克隆抗体对细胞进行染色(Sharma等人,2012)。简而言之,将淋巴细胞(2.5×106)与malabaricone C (mal C)(10μM,2小时)一起孵育,然后用Con A刺激(2.5μg/mL,24小时用于表面染色,2.5μg/mL,2小时用于细胞内染色),随后用荧光染料偶联的CD69或CD25单克隆抗体染色。使用未染色和同种型染色的细胞作为对照。然后,在BD FACS Melody流式细胞仪上采集细胞,并使用FlowJo软件进行分析。 ROS和GSH的估算[2] 对于基于DCF荧光(485/535nm处的激发/发射)的ROS估计,用H2DCF-DA对malabaricone C (mal C)或载体处理的细胞进行染色,并使用光谱荧光计 进行监测。为了定量GSH(还原/氧化)水平,用Mal C或载体对照处理细胞,收获并裂解,并如前所述探测细胞提取物的GSH定量(Patwardhan等人,2015)。为了评估细胞巯基,细胞用MCB染色(在390/478 nm处激发/发射),然后进行流式细胞术采集。 高效液相色谱法分析malabaricone C (mal C)和NAC相互作用[2] 我们采用多步梯度流动相,乙腈为溶剂a,甲酸水溶液(0.1%)为溶剂B,使用C-18柱进行色谱分离,并采用优化的方法。梯度设置为:40%溶剂A 2分钟;80%溶剂A处理22分钟;100%溶剂A浸泡2分钟;40%溶剂A 4分钟,流速保持在1 mL/min。注射用样品体积为20μL,总运行时间为30分钟,在15.773分钟的保留时间(Rt)下检测Mal C。使用二极管阵列检测器在274 nm下进行检测。制备100 mM浓度的NAC储备溶液,并在1X PBS中稀释。将Mal C加入NAC中(Mal C 100μM+NAC 100µM),在37°C下孵育1小时,并在HPLC上运行(Dionex Ultima 3000系列HPLC系统,带Chromeleon软件(版本6.8))。 malabaricone C (mal C)和NAC相互作用的光谱分析[2] Mal C(100μM)与NAC(100μM)在37°C的水中孵育1小时,使用Jasco分光光度计记录250至700 nm的吸收光谱。使用Jasco Spectra Manager Ver.2软件绘制了显示吸光度与波长(nm)的图。 蛋白质印迹[2] 收获用malabaricone C (mal C)或Con A(2.5μg/mL)或两者处理的细胞(10×106/mL),并使用1X RIPA裂解缓冲液进行裂解,该缓冲液由50 mM Tris-HCl、150 mM NaCl、1.0%(v/v)NP-40、0.5%(w/v)脱氧胆酸钠、1.0 mM EDTA、0.1%(w/v。使用Bradford测定法进行蛋白质估算,然后在SDS-PAGE(10%)上进行电泳分离。将蛋白质转移到PVDF膜上,使用单克隆抗体和HRP偶联的二抗探测pERK、pJNK、ERK和JNK。使用增强化学发光试剂盒(POD)在SynGene:GBox凝胶记录系统中可视化条带。 转录因子凝胶转移分析[2] 细胞(10×106/mL)用载体或指定浓度的malabaricone C (mal C)或Con A(2.5μg/mL)或两者处理。如前所述(Patwardhan等人,20152016),从细胞中制备核提取物,并与来自人类免疫缺陷病毒长末端重复序列的32P末端标记的45-mer双链NF-κB寡核苷酸(5′-TTGTTACAGGGACTTTCCGTGGGGACTTCGGGAGGCGTG-3′)一起孵育。样品在天然聚丙烯酰胺凝胶上运行,然后干燥凝胶,随后将其暴露于荧光图像板上,使用荧光图像板扫描仪观察放射性带。 |
| 动物实验 |
Ex vivo stimulation of lymphocytes [2]
The high lipophilicity and low water solubility of malabaricone C (mal C) leads to poor oral bioavailability, and hence mice were administered with malabaricone C (mal C) (10 mg/kg body weight) or vehicle intra-peritoneally (3 mice per group) and were sacrificed 24 h after the injection. Lymphocytes were stimulated with Con A (2.5 μg/mL) for 24 h for estimation of secreted cytokines using ELISA. Another set of lymphocytes was stained with CFSE and stimulated with Con A (2.5 μg/mL) for 72 h for assessment of cell proliferation by flow cytometry. Graft-versus-host disease [2] Recipient BALB/c mice were exposed to whole-body irradiation (WBI) of 6 Gy at a dose rate of 1 Gy/min in a blood irradiator for induction of lymphopenia (6 mice per group). The lymphocytes from allogenic C57BL/6 donors were treated with vehicle control or malabaricone C (mal C) in vitro, and 10 million cells were administered intravenously into the lateral tail vein of lymphopenic recipients 48 h after WBI. After engraftment, lymphopenic recipient mice were monitored for assessment of changes in the body weight and survival up to 30 days. On day 5 post engraftment, blood was collected from lymphopenic recipient mice and the serum was separated to monitor the cytokine levels using ELISA. Monitoring homeostatic proliferation in vivo [2] CD4+ T-cells were sorted from lymphocytes of BALB/c mice using magnetic beads and were stained with CFSE. The cells were incubated with malabaricone C (mal C) (10 μM) or vehicle for 4 h at 37°C in 5% CO2. The cells were washed and 1 million CFSE+ CD4+ T-cells were intravenously engrafted into lymphopenic syngeneic BALB/c mice (3 mice per group). After 72 h, lymphocytes from lymphopenic recipient mice were isolated and the frequency and proliferation of donor CFSE+ cells were monitored by flow cytometry. |
| 参考文献 |
|
| 其他信息 |
malabarine C(mal C) is a butanone. It has a role as a metabolite.
Malabaricone C has been reported in Myristicaceae, Myristica fragrans, and other organisms with data available. In summary, malabarine C(mal C), an acylphenol isolated from the fruits of M. cinnamomea, has been identified as a lead natural sphingomyelin synthase inhibitor. Having the same mechanisms of action as the previously reported SMS knockout studies, malabaricone C was highly efficacious in preventing oleic acid uptake across the membrane, which in turn reduced lipid droplet formation in vitro.15 Malabaricone C was also found to be able to reduce body weight gain, improve glucose tolerance, and decrease lipid accumulation in the liver in vivo, thus making this the first report involving a plant derived SMS inhibitor against high fat diet-induced obesity. Its nontoxic nature makes malabaricone C a suitable candidate for its further development as a new drug or medicinal supplement to treat and prevent obesity. [1] T-cells are important mediators of adaptive immune responses. However, under specific pathological conditions like autoimmunity, allergy, chronic inflammation, COVID-19, and acute GvHD, there is a need to suppress T-cell activation (Maurice et al. 1997; Griffiths et al. 2011; Weyand et al. 2018). The past few decades have seen unprecedented research on the development of prophylactic as well as therapeutic agents to suppress undesirable T-cell activation in the clinic. Many researchers, including our group, have earlier highlighted the influence of cellular redox status and surrounding redox environment in the control of T-cell activation, proliferation, and differentiation (Checker et al. 2009; Kesarwani et al. 2013; Gambhir et al. 2014). Many redox active agents including antioxidants as well as pro-oxidants have been shown to modulate T-cell activation. Previously we demonstrated that pro-oxidants like plumbagin, 1,4-naphthoquinone, and menadione could inhibit T-cell activation at sub-micromolar concentrations through the modulation of GSH levels in cells (Checker et al. 2010, 2011). Antioxidants like GSH, NAC, chlorophyllin, baicalein, and vitamin C have been shown to modulate the activation and differentiation of T-cells (Sharma et al. 2007; Patwardhan et al. 2016). GSH dysregulation is also implicated in early experimental GvHD severity (Suh et al. 2014). Activation of T-cells is also dependent on the intrinsic generation of ROS in the mitochondria and metabolic reprogramming (Sena et al. 2013). Bombay mace, or false nutmeg, is a spice derived from the fruit aril of an Indian medicinal plant Myristica malabarica (Myristicaceae; Ayurvedic name, Rampatri) (Patro et al. 2005). Based on its chemical structure and the known biological properties of its active ingredient, we hypothesized that malabarine C(mal C) may affect T-cell activation. It was indeed found that malabarine C(mal C) treatment significantly suppressed the activation, proliferation, and cytokine secretion in murine T-cells. Further, Mal C treatment suppressed Con A-induced ROS generation. Here we observed that inhibition of T-cell activation was accompanied by a decrease in total ROS levels. Incubation of lymphocytes with Mal C resulted in significant depletion of reduced GSH levels. Hence, a question arises as to whether the efficacy of Mal C as an immune-modulatory agent is due to its antioxidant nature or due to its thiol-seeking behaviour. Interestingly, the anti-proliferative effects of Mal C were abrogated by thiol antioxidants, suggesting possible interaction of Mal C with cellular thiols. Indeed, biophysical studies revealed that Mal C could physically interact with NAC in an aqueous environment. Interestingly, NAC supplementation restored cellular thiol levels in Mal C-treated cells, which could be responsible for the abrogation of anti-proliferative effects of Mal C on T-cells in the presence of thiol antioxidants. These studies revealed that Mal C could possibly interact with cellular protein and non-protein thiols, thereby disrupting cellular redox. Cellular redox changes affect biological responses such as proliferation, differentiation, survival, and apoptosis through redox sensory signaling molecules (Chiu and Dawes 2012; Hancock and Whiteman 2018). MAP kinases can respond to cellular redox changes in terms of their phosphorylation status through the action of redox-sensitive phosphatases (Kamata and Hirata 1999; Seth and Rudolph 2006). T-cell receptor ligation induces distinct MAPK signaling that regulates T-cell responses (Adachi and Davis 2011). Mitogenic stimulation of T-cells leads to phosphorylation of MAP kinases, in turn leading to the activation of immune-regulatory transcription factor NF-κB. Mal C treatment suppressed phosphorylation of ERK and JNK and the subsequent DNA binding of NF-κB following mitogenic stimulation. Upon activation through signals delivered by the T-cell receptor and costimulatory molecules, T-cells upregulate the expression of CD25 (IL2Rα) and CD69 (c-type lectin). While CD25 expression determines IL2 responsiveness, CD69 activation stimulates an influx of calcium ions and the activation of extracellular kinases ERK1/2, thereby facilitating T-cell proliferation (Chen et al. 2020). Mal C-mediated inhibition of both early (CD69) as well as late activation marker (CD25) in T-cells indicates that Mal C interferes with the early activation of T-cells and renders them unresponsive to mitogenic stimulation. Based on these results, we hypothesized that transient exposure of T-cells to Mal C may be beneficial for prophylaxis of acute GvHD. We used a murine model of complete allogenic lymphocyte transplantation and found that Mal C completely abrogated acute GvHD-associated morbidity and mortality of the lymphopenic hosts. Mal C treatment of donor T-cells also resulted in significant reduction in GvHD-associated serum cytokines in the host. In vivo suppression of T-cell proliferation can also result in the disruption of immune homeostasis. In order to study the effect of Mal C on the behaviour of syngeneic T-cells, we evaluated the homeostatic proliferation of purified CD4+ T-cells in syngeneic lymphopenic hosts. Mal C did not inhibit the homeostatic proliferation of T-cells. Mal C treatment led to non-classical cellular redox perturbation by simultaneous scavenging of ROS and thiols. However, glutathione is essential for antigen-induced T-cell proliferation but not for homeostatic proliferation (Sena and Chandel 2012; Sena et al. 2013). Hence, it is anticipated that Mal C treatment did not affect the homeostatic proliferation of T-cells. [2] The signaling requirements for antigen-specific proliferation of T-cells during acute GvHD are different from those involved in homeostatic proliferation in response to lymphopenia. Our results establish a differential and specific inhibitory action of malabarine C(mal C) on antigen/mitogen-induced proliferation but not on the homeostatic proliferation of T-cells. [2] In summary, this is the first report showing inhibition of T-cell activation, proliferation, and cytokine production by malabarine C(mal C) through modulation of cellular redox balance (figure 5). Since Mal C suppressed only mitogen/alloantigen-induced proliferation but not homeostatic proliferation, it may be useful for prophylaxis of acute GvHD without disruption of the host immune reconstitution. The present results warrant further mechanistic studies to elucidate the molecular mechanism and biochemical targets of Mal C in T-cells.[2] |
| 分子式 |
C21H26O5
|
|---|---|
| 分子量 |
358.42814
|
| 精确质量 |
358.178
|
| 元素分析 |
C, 70.37; H, 7.31; O, 22.32
|
| CAS号 |
63335-25-1
|
| PubChem CID |
100313
|
| 外观&性状 |
White to off-white solid powder
|
| 熔点 |
119 - 121 °C
|
| LogP |
4.665
|
| tPSA |
97.99
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
10
|
| 重原子数目 |
26
|
| 分子复杂度/Complexity |
403
|
| 定义原子立体中心数目 |
0
|
| SMILES |
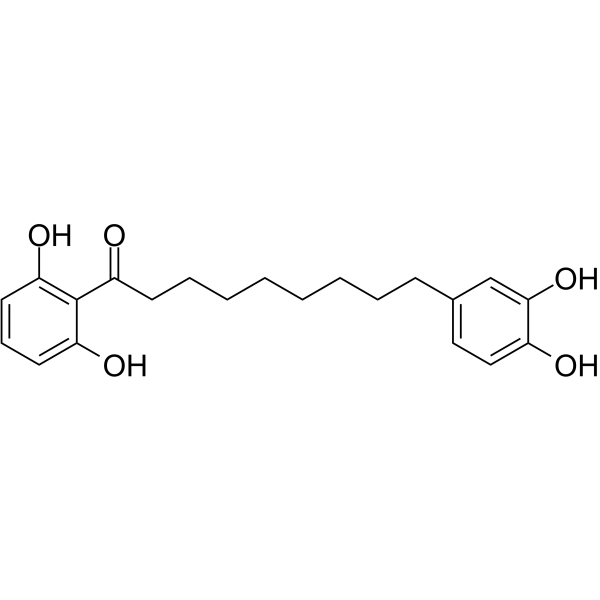
C1=CC(=C(C(=C1)O)C(=O)CCCCCCCCC2=CC(=C(C=C2)O)O)O
|
| InChi Key |
HCOZRFYGIFMIEX-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C21H26O5/c22-16-13-12-15(14-20(16)26)8-5-3-1-2-4-6-9-17(23)21-18(24)10-7-11-19(21)25/h7,10-14,22,24-26H,1-6,8-9H2
|
| 化学名 |
1-(2,6-dihydroxyphenyl)-9-(3,4-dihydroxyphenyl)nonan-1-one
|
| 别名 |
Malabaricone C; 63335-25-1; 1-(2,6-dihydroxyphenyl)-9-(3,4-dihydroxyphenyl)nonan-1-one; CHEBI:69015; C9K53R3PRN; DTXSID40212721; NSC 287968; NSC-287968;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~278.99 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.97 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.97 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.97 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.7899 mL | 13.9497 mL | 27.8995 mL | |
| 5 mM | 0.5580 mL | 2.7899 mL | 5.5799 mL | |
| 10 mM | 0.2790 mL | 1.3950 mL | 2.7899 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。