| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
The target of Maslinic acid is the nuclear factor kappa B (NF-κB) signaling pathway. It inhibits the activation of the NF-κB pathway [1]
|
|---|---|
| 体外研究 (In Vitro) |
山楂酸已被证明可以控制 IκB-α 的磷酸化和 LPS 诱导的 NF-κB 从细胞到细胞核的易位。据报道,山楂酸可降低 TNF-α 诱导的 NF-κB 活性及其下游基因在胰腺中的表达,并调节支架单核细胞中 NF-κB 调节的破骨细胞生成。需要确定山楂酸有效浓度为 10 -20 μM 的实验剂量,以确认橄榄果渣提取物 (OPE) 在 RAW264.7 细胞中的抗炎活性是否能够最终消除山楂酸。在 RAW 264.7 细胞中,20 μM 山楂酸显着降低 COX-2、IL-1 和 IL-6 mRNA 的表达以及 TNF-α 的产生。在 RAW 264.7 细胞中,LPS 引起的乳腺酸(10 和 20 μM)显着降低 NF-κB p65 的 DNA 结合活性。山楂酸能够显着降低 LPS 诱导的 IκB-α 磷酸化 [1]。
1. 在脂多糖(LPS)激活的RAW264.7巨噬细胞中,山楂酸呈剂量依赖性抑制促炎介质的产生。浓度为25 μM和50 μM时,与仅LPS处理组相比,分别使肿瘤坏死因子-α(TNF-α)mRNA水平降低约45%和70%、白细胞介素-6(IL-6)mRNA水平降低约40%和65%、诱导型一氧化氮合酶(iNOS)mRNA水平降低约35%和60%;对应的TNF-α、IL-6、iNOS蛋白水平也在这些浓度下降低30%-65% [1] 2. 山楂酸可抑制LPS刺激的RAW264.7细胞中NF-κB通路的活化。25 μM和50 μM 山楂酸处理分别使NF-κB抑制蛋白α(IκBα)的磷酸化水平降低约40%和65%,并抑制NF-κB p65亚基的核转位。免疫荧光结果显示,药物处理组细胞中p65的核积累量减少50%-70% [1] 3. MTT实验表明,山楂酸浓度在25 μM至100 μM范围内,对RAW264.7细胞无显著细胞毒性,细胞活力保持在90%以上 [1] |
| 体内研究 (In Vivo) |
当动物在注射 λ-角叉菜胶四小时后给予 200 mg/kg 山楂酸时,与角叉菜胶诱导的支架相比,它们的爪子肿胀减少(分别为 0.91 ± 0.51 mm 和 1.79 ± 0.4 mm [1]) 。
1. 在胶原诱导关节炎(CIA)小鼠模型中,口服给予山楂酸(20 mg/kg、40 mg/kg,每日一次,连续21天)可显著缓解关节炎症状。与CIA模型组相比,该剂量分别使关节炎评分(基于爪肿胀和发红程度)降低约35%(20 mg/kg)和55%(40 mg/kg),爪体积分别减少30%和50% [1] 2. 山楂酸可改善CIA小鼠的关节组织病理状态。组织学分析显示,40 mg/kg 山楂酸处理后,与模型组相比,滑膜增生、炎症细胞浸润和软骨破坏程度分别减少约60%、55%和50% [1] 3. 在CIA小鼠血清中,山楂酸(20 mg/kg、40 mg/kg)可降低促炎细胞因子水平:TNF-α分别降低35%和60%、IL-6分别降低30%和55%、IL-1β分别降低25%和50%。此外,关节组织中NF-κB通路活化受抑制,表现为p-IκBα和核内p65水平降低 [1] |
| 酶活实验 |
NF-κB报告基因检测实验:将HEK293细胞接种于24孔板,培养至融合度70%。使用转染试剂将NF-κB-荧光素酶报告质粒与海肾荧光素酶质粒(内参)共转染至细胞。转染24小时后,用山楂酸(12.5 μM、25 μM、50 μM)或二甲基亚砜(DMSO,对照)预处理细胞1小时,再用肿瘤坏死因子-α(TNF-α,10 ng/mL)刺激6小时。裂解细胞后,采用双荧光素酶报告基因检测系统测定荧光素酶活性,通过计算相对荧光素酶活性(NF-κB荧光素酶/海肾荧光素酶)评估山楂酸对NF-κB活化的抑制作用 [1]
|
| 细胞实验 |
1. RAW264.7巨噬细胞培养与处理:将RAW264.7细胞置于含10%胎牛血清和1%抗生素的完全培养基中,在37°C、5% CO2培养箱中培养。将细胞接种于6孔板(用于mRNA/蛋白检测)或96孔板(用于MTT实验),贴壁24小时后,用山楂酸(12.5 μM、25 μM、50 μM)或DMSO预处理1小时,再用LPS(1 μg/mL)刺激24小时(用于细胞因子检测)或15分钟(用于NF-κB通路蛋白检测) [1]
2. MTT细胞活力实验:处理结束后,向96孔板每孔加入20 μL MTT溶液(5 mg/mL),37°C孵育4小时。弃去上清液,加入150 μL DMSO溶解甲臜结晶,用酶标仪测定570 nm处吸光度,以对照组为基准计算细胞活力百分比 [1] 3. 定量实时PCR(qPCR):用RNA提取试剂盒提取RAW264.7细胞总RNA,通过逆转录合成cDNA,使用TNF-α、IL-6、iNOS及内参基因GAPDH的特异性引物进行qPCR,采用2^(-ΔΔCt)法计算相对mRNA表达量 [1] 4. Western blot检测:提取细胞总蛋白,用蛋白定量试剂盒测定蛋白浓度。将等量蛋白通过SDS-PAGE电泳分离,转移至PVDF膜,用5%脱脂牛奶封闭1小时。膜与抗TNF-α、IL-6、iNOS、IκBα、p-IκBα、NF-κB p65及内参GAPDH的一抗在4°C孵育过夜,再与二抗室温孵育1小时。用增强化学发光试剂盒显影条带,通过图像分析软件定量条带灰度值 [1] 5. NF-κB p65免疫荧光实验:将RAW264.7细胞接种于盖玻片,按上述方法处理后,用4%多聚甲醛固定、0.1% Triton X-100透化,1%牛血清白蛋白(BSA)封闭。加入抗NF-κB p65一抗4°C孵育过夜,再加入荧光二抗和DAPI(细胞核染色)室温孵育1小时。封片后,在荧光显微镜下观察p65的定位情况 [1] |
| 动物实验 |
1. Collagen-induced arthritis (CIA) model establishment: DBA/1 mice (male, 6-8 weeks old) were immunized by intradermal injection of 100 μg of bovine type II collagen emulsified in complete Freund's adjuvant at the base of the tail. A booster injection of 50 μg of type II collagen in incomplete Freund's adjuvant was given on day 21 after the first immunization [1]
2. Grouping and drug administration: Mice were divided into four groups (n=6 per group): normal control group (no immunization, no drug), CIA model group (immunized, oral administration of 0.5% carboxymethyl cellulose sodium (CMC-Na)), Maslinic acid low-dose group (immunized, oral administration of 20 mg/kg Maslinic acid dissolved in 0.5% CMC-Na), and Maslinic acid high-dose group (immunized, oral administration of 40 mg/kg Maslinic acid dissolved in 0.5% CMC-Na). Drug administration started on day 22 after the first immunization and continued once daily for 21 days [1] 3. Sample collection and analysis: During the experiment, paw volume was measured using a plethysmometer, and arthritis score was evaluated every 3 days. On day 43 (end of treatment), mice were anesthetized, and blood was collected from the heart to separate serum for cytokine detection. Hind paw joints were removed, fixed in 4% paraformaldehyde, decalcified, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E) for histological analysis. Joint tissues were also used for Western blot to detect NF-κB pathway-related proteins [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
1. In vivo toxicity: In CIA mice treated with Maslinic acid (20 mg/kg and 40 mg/kg, oral, 21 days), no significant changes in body weight were observed compared to the normal control group. Serum levels of alanine transaminase (ALT), aspartate transaminase (AST), blood urea nitrogen (BUN), and creatinine (Cr) were within the normal range, indicating no obvious liver or kidney toxicity [1]
2. In vitro toxicity: MTT assay showed that Maslinic acid at concentrations up to 100 μM had no significant cytotoxicity on RAW264.7 cells, with cell viability maintained above 90% [1] |
| 参考文献 | |
| 其他信息 |
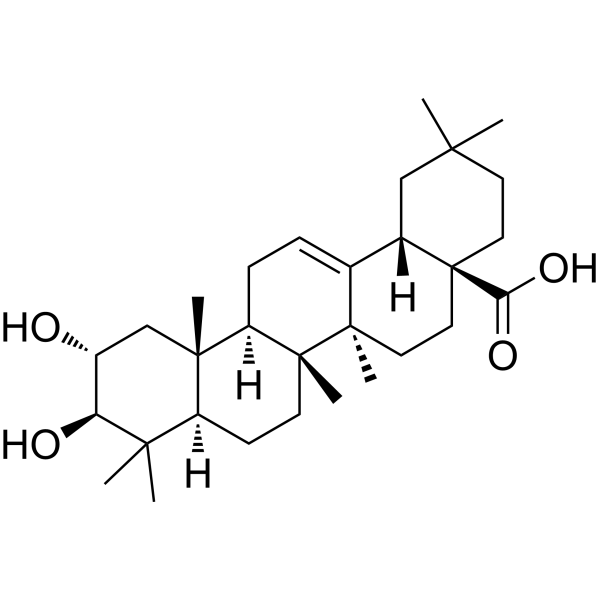
Maslinic acid is a pentacyclic triterpenoid that is olean-12-ene substituted by hydroxy groups at positions 2 and 3 and a carboxy group at position 28 (the 2alpha,3beta stereoisomer). It is isolated from Olea europaea and Salvia canariensis and exhibits anti-inflammatory, antioxidant and antineoplastic activity. It has a role as an antioxidant, an antineoplastic agent, an anti-inflammatory agent and a plant metabolite. It is a pentacyclic triterpenoid and a dihydroxy monocarboxylic acid. It derives from a hydride of an oleanane.
Maslinic acid has been reported in Salvia miltiorrhiza, Sideritis candicans, and other organisms with data available. See also: Centaurium erythraea whole (part of). Mechanism of Action Maslinic acid, a pentacyclic triterpene found in the protective wax-like coating of the leaves and fruit of Olea europaea L., is a promising agent for the prevention of colon cancer. Investigators have shown /previously/ that maslinic acid inhibits cell proliferation to a significant extent and activates mitochondrial apoptosis in colon cancer cells. /This study/ investigated... this compound's apoptotic molecular mechanism. /Investigators/ used HT29 adenocarcinoma cells. Changes /in/ genotoxicity were analyzed by single-cell gel electrophoresis (comet assay). The cell cycle was determined by flow cytometry. Finally, changes in protein expression were examined by western blotting. Student's t-test was used for statistical comparison. HT29 cells treated with maslinic acid showed significant increases in genotoxicity and cell-cycle arrest during the G0/G1 phase after 72 hours treatment and an apoptotic sub-G0/G1 peak after 96 hours... the anti-tumoral activity of maslinic acid might proceed via p53-mediated apoptosis by acting upon the main signaling components that lead to an increase in p53 activity and the induction of the rest of the factors that participate in the apoptotic pathway. /Investigators/ found that in HT29 cells maslinic acid activated the expression of c-Jun NH2-terminal kinase (JNK), thus inducing p53. Treatment of tumor cells with maslinic acid also resulted in an increase in the expression of Bid and Bax, repression of Bcl-2, release of cytochrome-c and an increase in the expression of caspases -9, -3, and -7. Moreover, maslinic acid produced belated caspase-8 activity, thus amplifying the initial mitochondrial apoptotic signaling. All these results suggest that maslinic acid induces apoptosis in human HT29 colon-cancer cells through the JNK-Bid-mediated mitochondrial apoptotic pathway via the activation of p53... Maslinic acid (2-alpha, 3-beta-dihydroxyolean-12-en-28-oic acid) is a natural triterpenoid compound from Olea europaea. This compound prevents oxidative stress and pro-inflammatory cytokine generation in vitro. This study ...investigated the anti-inflammatory effects of maslinic acid in central nervous system by using rat astrocyte cultures stimulated with lipopolysaccharide (LPS). /It/ evaluated different proteins implicated in the nuclear factor kappa B (NF-kappa B) signal transducer pathway employing Western blot and quantitative real time PCR techniques. Results demonstrated that maslinic acid treatment exerted potent anti-inflammatory action by inhibiting the production of Nitric Oxide and tumor necrosis factor alpha (TNF-alpha). Western blot analysis showed that maslinic acid treatment attenuated LPS-induced translocation of NF-kappa B p65 subunit to the nucleus and prevented LPS-induced I kappa B alpha phosphorylation in a concentration-dependent manner, Moreover, maslinic acid significantly suppressed the expression of cyclooxygenase 2 (COX-2) and inducible nitric oxide synthase (iNOS) at protein and mRNA levels. These results suggest that maslinic acid can potentially reduce neuroinflammation by inhibiting NF-kappa B signal transducer pathway in cultured cortical astrocytes. Activation of NF-kappaB and MAPK/activator protein 1 (AP-1) signaling pathways by receptor activator NF-kappaB ligand (RANKL) is essential for osteoclast activity. Targeting NF-kappaB and MAPK/AP-1 signaling to modulate osteoclast activity has been a promising strategy for osteoclast-related diseases. /This study/ examined the effects of maslinic acid (MA), a pentacyclic triterpene acid that is widely present in dietary plants, on RANKL-induced osteoclastogenesis, osteoclast function, and signaling pathways by in vitro and in vivo assay systems. In mouse bone marrow monocytes (BMMs) and RAW264.7 cells, MA inhibited RANKL-induced osteoclastogenesis in a dose-dependent manner within nongrowth inhibitory concentration, and MA decreased osteoclastogenesis-related marker gene expression, including TRACP, MMP9, c-Src, CTR, and cathepsin K. Specifically, MA suppressed osteoclastogenesis and actin ring formation at early stage. In ovariectomized mice, administration of MA prevented ovariectomy-induced bone loss by inhibiting osteoclast activity. At molecular levels, MA abrogated the phosphorylation of MAPKs and AP-1 activity, inhibited the Ikappa Balpha phosphorylation and degradation, blocked NF-kappaB/p65 phosphorylation, nuclear translocation, and DNA-binding activity by downregulating RANK expression and blocking RANK interaction with TRAF6. Together /this/ data demonstrate that MA suppresses RANKL-induced osteoclastogenesis through NF-kappa B and MAPK/AP-1 signaling pathways and that MA is a promising agent in the treatment of osteoclast-related diseases such as osteoporosis. 1. Maslinic acid is a pentacyclic triterpenoid primarily isolated from olive peel extracts. Its anti-inflammatory and anti-arthritic effects are mainly mediated by inhibiting the NF-κB signaling pathway—specifically by reducing IκBα phosphorylation and preventing NF-κB p65 nuclear translocation, thereby suppressing the production of proinflammatory cytokines and mediators [1] 2. The study demonstrated that Maslinic acid alleviates collagen-induced arthritis in mice without obvious toxicity, suggesting its potential as a candidate for the development of anti-inflammatory or anti-arthritic agents [1] |
| 分子式 |
C30H48O4
|
|---|---|
| 分子量 |
472.6997
|
| 精确质量 |
472.355
|
| CAS号 |
4373-41-5
|
| PubChem CID |
73659
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
570.0±50.0 °C at 760 mmHg
|
| 熔点 |
249 - 250 °C
|
| 闪点 |
312.6±26.6 °C
|
| 蒸汽压 |
0.0±3.6 mmHg at 25°C
|
| 折射率 |
1.568
|
| LogP |
7.87
|
| tPSA |
77.76
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
34
|
| 分子复杂度/Complexity |
919
|
| 定义原子立体中心数目 |
9
|
| SMILES |
C[C@@]12CC[C@@H]3[C@@]([C@H]1CC=C4[C@]2(CC[C@@]5([C@H]4CC(CC5)(C)C)C(=O)O)C)(C[C@H]([C@@H](C3(C)C)O)O)C
|
| InChi Key |
MDZKJHQSJHYOHJ-LLICELPBSA-N
|
| InChi Code |
InChI=1S/C30H48O4/c1-25(2)12-14-30(24(33)34)15-13-28(6)18(19(30)16-25)8-9-22-27(5)17-20(31)23(32)26(3,4)21(27)10-11-29(22,28)7/h8,19-23,31-32H,9-17H2,1-7H3,(H,33,34)/t19-,20+,21-,22+,23-,27-,28+,29+,30-/m0/s1
|
| 化学名 |
(4aS,6aR,6aS,6bR,8aR,10R,11R,12aR,14bS)-10,11-dihydroxy-2,2,6a,6b,9,9,12a-heptamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~211.55 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.5 mg/mL (5.29 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.29 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1155 mL | 10.5775 mL | 21.1551 mL | |
| 5 mM | 0.4231 mL | 2.1155 mL | 4.2310 mL | |
| 10 mM | 0.2116 mL | 1.0578 mL | 2.1155 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。