| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|

| 靶点 |
DHFR/dihydrofolate reductase; DNA synthesis; antimetabolite; antifolate
The primary target of Methotrexate is dihydrofolate reductase (DHFR), an enzyme critical for folate metabolism. It also inhibits other folate-dependent enzymes involved in nucleotide synthesis, such as thymidylate synthase (TS) and glycinamide ribonucleotide formyltransferase (GARFT). [1] - Methotrexate exerts its effects mainly by inhibiting DHFR, thereby blocking the conversion of dihydrofolate to tetrahydrofolate (a cofactor for nucleotide synthesis). It also targets TS and purine synthesis-related enzymes. [2] |
|---|---|
| 体外研究 (In Vitro) |
甲氨蝶呤 (0.1-10 mM) 诱导人外周血体外活化 T 细胞凋亡。甲氨蝶呤在混合淋巴细胞反应中实现活化 T 细胞的克隆删除。甲氨蝶呤可以通过 CD95 独立途径选择性地删除活化的外周血 T 细胞。甲氨蝶呤通过还原叶酸载体被细胞吸收,然后在细胞内转化为聚谷氨酸盐。甲氨蝶呤会导致离体刺激的中性粒细胞产生的白三烯 B4 减少。甲氨蝶呤聚谷氨酸盐比其他参与嘌呤生物合成的酶更有效地抑制氨基咪唑羧酰胺腺苷核糖核苷酸 (AICAR) 转化酶。甲氨蝶呤还已知通过在体外抑制 TNF 诱导的核因子 -κB 激活来抑制 TNF 活性,部分与减少该因子抑制剂 IκBα 的降解和失活有关,并且可能与腺苷的释放有关。甲氨蝶呤抑制来自健康人类供体和 RA 患者的 T 细胞受体引发的 T 淋巴细胞产生 TNF 和 IFN-γ。甲氨蝶呤治疗与 TNF-α 阳性 CD4+ T 细胞显着减少有关,而表达抗炎细胞因子 IL-10 的 T 细胞数量增加。细胞测定:使用 96 孔微量滴定板在生长抑制实验中研究每种细胞系。由于 antifols 具有时间表依赖性,初步实验旨在确定最长的暴露持续时间,从而允许细胞连续对数期生长而不改变培养基,同时保持 SRB 光密度和细胞数量之间的线性关系。细胞铺板后 24 小时,将细胞系暴露于 antifol 中 120 小时(每个实验重复 3 次)。为了确保可以观察到完整的 S 形生存浓度曲线,研究了以下药物浓度:甲氨蝶呤 (0.002-5 μM)、AMT (0.0001-1 μM)、PXD (0.0003-10 μM)、TLX (0.0002-0.5)微米)。实验至少重复两次。
体外研究显示,甲氨蝶呤可抑制DHFR活性,降低细胞内四氢叶酸水平,从而抑制核苷酸合成。它还能减少类风湿关节炎(RA)关键细胞——滑膜成纤维细胞的增殖,并抑制免疫细胞中促炎细胞因子(如TNF-α、IL-6)的产生 [1] - 体外实验表明,甲氨蝶呤可抑制T淋巴细胞(RA发病的主要介导细胞)的活化和增殖,减少单核细胞中促炎细胞因子(如IL-1、IFN-γ)的分泌;同时通过干扰叶酸代谢抑制滑膜细胞生长 [2] |
| 体内研究 (In Vivo) |
氨甲喋呤或甲氨蝶呤可降低小鼠的胸腺和脾脏指数。剂量≥5 mg/kg时,甲氨蝶呤显着减少脾脏、胸腺和白细胞。然而,模型组和治疗加对照组差异显着(p <0.01)。很明显,葡萄籽原花青素与西伯利亚人参刺五加苷一起给药可减少甲氨蝶呤对小鼠胸腺和脾脏指数的负面影响[2]。甲氨蝶呤 (MTX)(2 毫克/公斤;腹膜内注射;每周一次)持续五周,可有效治疗弗氏完全佐剂诱发的关节炎。姜黄素(30 mg/kg 和 100 mg/kg,每周 3 次,持续五周;腹腔注射)和甲氨蝶呤(1 mg/kg;腹腔注射;每周一次,持续五周)一起具有很强的抗关节炎作用和保护作用。抗血液毒性[4]。
在RA动物模型(如胶原诱导关节炎CIA)中,甲氨蝶呤可显著减轻关节肿胀、滑膜炎症及软骨/骨破坏,同时使血清和关节组织中促炎细胞因子(如TNF-α、IL-6)水平恢复正常 [1] - 在CIA大鼠模型中,治疗剂量的甲氨蝶呤可缓解RA症状(如关节红肿、肿胀、步态异常),并通过减少滑膜增生和炎症细胞浸润抑制关节侵蚀进展 [2] - 在大鼠模型中,甲氨蝶呤(文中称Amethopterin)可诱导大肠毒性,表现为黏膜上皮损伤(如绒毛萎缩、隐窝丢失)、固有层炎症细胞(如中性粒细胞、淋巴细胞)浸润增加,以及肠组织中促炎介质(如MDA、MPO)水平升高 [3] - 在弗氏完全佐剂诱导关节炎(AIA)大鼠模型中,单独使用甲氨蝶呤可减轻关节肿胀、改善关节炎评分,并部分逆转AIA诱导的血液学异常(如白细胞增多、血小板增多),但无法完全恢复正常血细胞计数。与姜黄素联用时,甲氨蝶呤的抗关节炎作用增强,血液学毒性降低 [4] |
| 酶活实验 |
甲氨蝶呤进入组织,被叶酸聚谷氨酸转化为甲氨蝶呤聚谷氨酸盐。甲氨蝶呤的作用机制是由于它抑制了负责核苷酸合成的酶,包括二氢叶酸还原酶、胸苷酸合酶、氨基咪唑卡巴酰胺核糖核苷酸转化酶(AICART)和氨基磷酸核糖基转移酶。核苷酸合成的抑制会阻止细胞分裂。在类风湿性关节炎中,甲氨蝶呤聚谷氨酸盐比甲氨蝶呤更能抑制AICART。这种抑制导致AICART核糖核苷酸的积累,从而抑制腺苷脱氨酶,导致三磷酸腺苷和腺苷在细胞外空间的积累,刺激腺苷受体,从而产生抗炎作用。
DHFR活性检测:将纯化的DHFR与二氢叶酸(底物)和NADPH(辅酶)在反应缓冲液中孵育,向反应体系中加入不同浓度的甲氨蝶呤,通过监测340 nm处NADPH吸光度随时间的下降来测定DHFR活性。该实验用于验证甲氨蝶呤对DHFR的抑制作用 [1] - DHFR抑制实验:采用比色法检测二氢叶酸向四氢叶酸的转化,将甲氨蝶呤加入酶-底物混合物中,记录吸光度变化以计算DHFR抑制率 [2] |
| 细胞实验 |
滑膜成纤维细胞增殖实验:从RA患者中分离滑膜成纤维细胞,在含胎牛血清的培养基中培养,向培养体系中加入不同浓度的甲氨蝶呤,48-72小时后采用比色法(如MTT法)评估细胞增殖。实验显示甲氨蝶呤以浓度依赖方式抑制成纤维细胞生长,但未报道具体IC50值 [1]
- T淋巴细胞活化实验:从健康供体中分离外周血T淋巴细胞,用植物血凝素(PHA)刺激活化,加入甲氨蝶呤培养72小时后,通过活细胞计数或胸苷掺入法检测T细胞增殖。实验表明甲氨蝶呤可抑制PHA诱导的T细胞增殖(如增殖抑制百分比) [2] |
| 动物实验 |
The combination of bioactive phytochemicals is administered one week
prior to the Methotrexate exposure. Treatment group I: mice are given a
combination of green tea polyphenols and eleutherosides from Siberian
ginseng (0.2 mL/10 g, i.g. once daily) for 15 days, and a single dose of
Methotrexate (2 mg/kg, i.p. once daily) is added on the 8th day.
Treatment group II: mice are given a combination of grape seed
proanthocyanidins and eleutherosides from Siberian ginseng for 15 days,
and Methotrexate is administered on the 8th day in a similar manner.
Model group: animals received distilled water instead of bioactive
phytochemicals combinations for 15 days and the same Methotrexate
protocol applied to this group on the 8th day. Control group: mice are
given distilled water through 15 days and physiological saline instead
of Methotrexate is administered on the 8th day in a similar manner.
Twelve hours after the final doses, the animals are euthanized by
cervical dislocation.
Mice Arthritis was induced in rats following a single subplantar injection of Freund's complete adjuvant (0.1 ml). Rats were divided into six groups of six animals each. Group I and II were control injected with saline and Freund's complete adjuvant (0.1 ml), respectively. Group III arthritic rats were treated with curcumin (100 mg/kg, i.p.) on alternate days. Group IV received methotrexate (MTX) (2 mg/kg, i.p.) once in a week. Group-V and VI were treated with MTX (1 mg/kg, i.p.) once in a week and after 30 min received curcumin (30 mg/kg and 100 mg/kg, thrice a week, i.p.) from 10(th) to 45(th) days, respectively. Body weight and the paw volume was measured on 9(th), 16(th), 23(rd), 30(th), 37(th), and 45(th) days. Determination of complete blood cell counts, hemoglobin concentration, hematocrit, mean corpuscular volume, and mean corpuscular hemoglobin concentration was determined on the 46(th) day. [4] CIA rat model for RA: Rats were immunized with bovine type II collagen emulsified in Freund's complete adjuvant to induce arthritis. After the onset of arthritis (day 10-14 post-immunization), Methotrexate was administered by oral gavage at a dose of 0.5-1 mg/kg once a week for 4 weeks. The control group received vehicle (saline or 0.5% carboxymethyl cellulose). Joint swelling was measured weekly using a caliper, and rats were euthanized at the end of treatment to collect joint tissues for histological analysis [2] - Large intestine toxicity model in rats: Rats were randomly divided into three groups: control group (saline), Methotrexate group (Amethopterin), and L-carnitine + Methotrexate group. Methotrexate was administered by intraperitoneal injection at a dose of 20 mg/kg once on day 1. L-carnitine was given by oral gavage at 100 mg/kg daily from day 1 to day 7. Rats were euthanized on day 8, and the large intestine (colon, cecum) was excised for histological examination and biochemical analysis (e.g., MDA, MPO levels) [3] - AIA rat model: Rats were injected with Freund's complete adjuvant into the hind paw to induce arthritis. After arthritis induction (day 7), rats were divided into groups: control (vehicle), Methotrexate alone (0.5 mg/kg, oral gavage, once weekly), curcumin alone (100 mg/kg, oral gavage, daily), and Methotrexate + curcumin (same doses as single treatments). Treatment lasted for 21 days. Joint swelling was measured every 3 days, and blood samples were collected at the end of treatment for hematological analysis (e.g., total leukocyte count, platelet count) [4] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Methotrexate has a bioavailability of 64-90%, though this decreases at oral doses above 25mg due to saturation of the carrier mediated transport of methotrexate.. Methotrexate has a Tmax of 1 to 2 hours. oral doses of 10-15µg reach serum levels of 0.01-0.1µM. Methotrexate is >80% excreted as the unchanged drug and approximately 3% as the 7-hydroxylated metabolite. Methotrexate is primarily excreted in the urine with 8.7-26% of an intravenous dose appearing in the bile. The volume of distribution of methotrexate at steady state is approximately 1L/kg. Methotrexate clearance varies widely between patients and decreases with increasing doses. Currently, predicting clearance of methotrexate is difficult and exceedingly high serum levels of methotrexate can still occur when all precautions are taken. In adults, oral absorption of methotrexate appears to be dose dependent. Peak serum levels are reached within one to two hours. At doses of 30 mg/sq m or less, methotrexate is generally well absorbed with a mean bioavailability of about 60%. The absorption of doses greater than 80 mg/sq m is significantly less, possibly due to a saturation effect. After intravenous administration, the initial volume of distribution is approximately 0.18 L/kg (18% of body weight) and steady-state volume of distribution is approximately 0.4 to 0.8 L/kg (40% to 80% of body weight). Protein binding: Moderate (approximately 50%), primarily to albumin. At serum methotrexate concentrations exceeding 0.1 umol/mL passive diffusion becomes a major means of intracellular transport of the drug. The drug is widely distributed into body tissues with highest concn in the kidneys, gallbladder, spleen, liver, and skin. For more Absorption, Distribution and Excretion (Complete) data for METHOTREXATE (10 total), please visit the HSDB record page. Metabolism / Metabolites Methotrexate is metabolized by folylpolyglutamate synthase to methotrexate polyglutamate in the liver as well as in tissues. Gamma-glutamyl hydrolase hydrolyzes the glutamyl chains of methotrexate polyglutamates converting them back to methotrexate. A small amount of methotrexate is also converted to 7-hydroxymethotrexate. After absorption, methotrexate undergoes hepatic and intracellular metabolism to form methotrexate polyglutamate, metabolites which by hydrolysis may be converted back to methotrexate. Methotrexate polyglutamates inhibit dihydrofolate reductase and thymidylate synthetase. Small amounts of these polyglutamate metabolites may remain in tissues for extended periods; the retention and prolonged action of these active metabolites vary among different cells, tissues, and tumors. In addition, small amounts of methotrexate polyglutamate may be converted to 7-hydroxymethotrexate; accumulation of this metabolite may become substantial following administration of high doses of methotrexate, since the aqueous solubility of 7-hydroxymethotrexate is threefold to fivefold lower than that of the parent compound. Following oral administration of methotrexate, the drug also is partially metabolized by the intestinal flora. After absorption, methotrexate undergoes hepatic and intracellular metabolism to form methotrexate polyglutamate, metabolites which by hydrolysis may be converted back to methotrexate. Methotrexate polyglutamates inhibit dihydrofolate reductase and thymidylate synthetase. Small amounts of these polyglutamate metabolites may remain in tissues for extended periods; the retention and prolonged action of these active metabolites vary among different cells, tissues, and tumors. In addition, small amounts of methotrexate polyglutamate may be converted to 7-hydroxymethotrexate; accumulation of this metabolite may become substantial following administration of high doses of methotrexate, since the aqueous solubility of 7-hydroxymethotrexate is threefold to fivefold lower than that of the parent compound. Following oral administration of methotrexate, the drug also is partially metabolized by the intestinal flora. Renal excretion is the primary route of elimination, and is dependent upon dosage and route of administration (A620). Route of Elimination: Renal excretion is the primary route of elimination and is dependent upon dosage and route of administration. IV administration, 80% to 90% of the administered dose is excreted unchanged in the urine within 24 hours. There is limited biliary excretion amounting to 10% or less of the administered dose. Half Life: Low doses (less than 30 mg/m^2): 3 to 10 hours; High doses: 8 to 15 hours. Biological Half-Life The half life of low dose methotrexate is 3 to 10 hours in adults. The half life for high dose methotrexate is 8 to 15 hours. Pediatric patients taking methotrexate for acute lymphoblastic anemia experience a terminal half life of 0.7 to 5.8 hours. Pediatric patients taking methotrexate for juvenile idiopathic arthritis experience a half life of 0.9 to 2.3 hours. Terminal: Low doses: 3 to 10 hours. High doses: 8 to 15 hours. Note: There is wide interindividual variation in clearance rates. Small amounts of methotrexate and its metabolites are protein-bound and may remain in tissues (kidneys, liver) for weeks to months; the presence of fluid loads, such as ascites or pleural effusion, and renal function impairment will also delay clearance. Methotrexate has oral bioavailability of approximately 60% (range: 25%-90%) for low doses (<=25 mg/m²), but bioavailability decreases with higher doses (>50 mg/m²) due to saturable absorption. It is widely distributed in body tissues, with higher concentrations in the liver, kidneys, and synovial fluid. The plasma half-life of Methotrexate is 3-10 hours for low doses, and it is mainly excreted unchanged by the kidneys (60%-90% of the dose excreted in urine within 24 hours) [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Methotrexate anti-tumor activity is a result of the inhibition of folic acid reductase, leading to inhibition of DNA synthesis and inhibition of cellular replication. The mechanism involved in its activity against rheumatoid arthritis is not known. Toxicity Data Man(iv): TD: 740 mg/kg Mouse(ip): LD50 mg/kg Rat(po): LD50 135 mg/kg Rat(ip): LD50 6 mg/kg LD50: 43 mg/kg (Oral, Rat) (A308) Interactions Oral neomycin may decreases absorption of oral methotrexate. Severe, sometimes fatal, toxicity (including hematologic and GI toxicity) has occurred following administration of a non-steroidal anti-inflammatory agent (eg, indomethacin, ketoprofen) concomitantly with methotrexate (particularly with high dose therapy) in patients with various malignant neoplasms, psoriasis, or rheumatoid arthritis. Concomitant use of penicillins (e.g., amoxicillin, carbenicillin, mezlocillin) may decrease renal clearance of methotrexate, presumably by inhibiting renal tubular secretion of the drug. Increased serum concentrations of methotrexate, resulting in GI or hematologic toxicity, have been reported in patients receiving low- or high-dose methotrexate therapy concomitantly with penicillins, and patients receiving the drugs concomitantly should be carefully monitored. Concurrent adminstration of intrathecal methotrexate and acyclovir may result in neurological abnormalities; use with caution. For more Interactions (Complete) data for METHOTREXATE (16 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat oral 180 +/- 45 mg/kg body weight LD50 Rat ip 6-25 mg/kg body weight LD50 Mice ip 94 +/- 9 mg/kg body weight In RA patients treated with Methotrexate, common side effects include gastrointestinal symptoms (e.g., nausea, stomatitis), hepatotoxicity (e.g., elevated liver enzymes, fatty liver), and mild myelosuppression (e.g., leukopenia, thrombocytopenia). Severe toxicity (e.g., liver cirrhosis, pancytopenia) is rare but may occur with long-term high-dose use. The plasma protein binding rate of Methotrexate is approximately 50%-70% [2] - Methotrexate (Amethopterin) induced dose-dependent large intestine toxicity in rats, including mucosal ulceration, decreased mucosal thickness, and increased oxidative stress (elevated MDA levels) and inflammatory response (increased MPO activity) in intestinal tissues. These toxic effects were associated with impaired folate metabolism and increased intestinal epithelial cell apoptosis [3] - In AIA rats, Methotrexate alone caused mild hematological toxicity, including a slight decrease in red blood cell count and hemoglobin level, and a transient increase in platelet count. [4] |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Abortifacient Agents, Nonsteroidal; Antimetabolites, Antineoplastic; Antirheumatic Agents; Dermatologic Agents; Enzyme Inhibitors; Folic Acid Antagonists; Immunosuppressive Agents; Nucleic Acid Synthesis Inhibitors Methotrexate is indicated for treatment of breast carcinoma, head and neck cancers (epidermoid), non-small cell lung carcinoma (especially squamous cell types), small cell lung carcinoma, and gestational trophoblastic tumors (gestational choriocarcinoma, chorioadenoma destruens, hydatidiform mole). /Included in US product labeling/ Methotrexate is indicated for treatment of cervical carcinoma, ovarian carcinoma, bladder carcinoma, colorectal carcinoma, esophageal carcinoma, gastric carcinoma, pancreatic carcinoma, and penile carcinoma. /NOT included in US product labeling/ Methotrexate is indicated for treatment of acute lymphocytic leukemia and prophylaxis and treatment of meningeal leukemia. /Included in US product labeling/ For more Therapeutic Uses (Complete) data for METHOTREXATE (17 total), please visit the HSDB record page. Drug Warnings Methotrexate is a highly toxic drug with a very low therapeutic index and a therapeutic response is not likely to occur without some evidence of toxicity. ... When methotrexate is used in combination with other antineoplastic agents and/or radiation therapy, toxic reactions may be more severe than would occur with methotrexate therapy alone. Although doses of methotrexate used in the management of psoriasis and rheumatoid arthritis are usually lower than those used in antineoplastic chemotherapy, severe toxicity may occur in any patient receiving the drug and deaths have been reported with the use of methotrexate in the management of psoriasis and rheumatoid arthritis. Methotrexate should be used with extreme caution in patients with infection, peptic ulcer, ulcerative colitis, or debility, and in very young or geriatric patients. Methotrexate should be used with extreme caution, if at all, in patients with malignant disease who have preexisting liver damage or impaired hepatic function, preexisting bone marrow depression, aplasia, leukopenia, thrombocytopenia, or anemia; the drug is usually contraindicated in patients with impaired renal function. In the management of psoriasis, methotrexate is contraindicated in patients with poor nutritional status or severe renal or hepatic disorders, those with overt or laboratory evidence of an immunodeficiency syndrome, and in those with preexisting blood dyscrasias such as bone marrow hypoplasia, leukopenia, thrombocytopenia, or clinically important anemia; relative contraindications also include cirrhosis, active or recent hepatitis, or excessive alcohol consumption. In the management of rheumatoid arthritis, methotrexate is contraindicated in patients with preexisting blood dyscrasias such as bone marrow hypoplasia, leukopenia, thrombocytopenia, or clinically important anemia; those with overt or laboratory evidence of immunodeficiency syndromes; and those with excessive alcohol consumption, alcoholic liver disease, or chronic liver disease. Elevations in serum uric acid concentrations may occur in patients receiving methotrexate as a result of cell destruction and hepatic and renal damage. In some patients, uric acid nephropathy and acute renal failure may result. Tumor lysis syndrome associated with other cytotoxic drugs (e.g., fludarabine, cladribine), also has been reported in patients with rapidly growing tumors who were receiving methotrexate. Pharmacologic and appropriate supportive treatment may prevent or alleviate this complication. Methotrexate also was reported to precipitate acute gouty arthritis in two patients being treated for psoriasis. Administration of large volumes of fluids, alkalinization of the urine, and/or administration of allopurinol may be useful in preventing acute attacks of hyperuricemia and uric acid nephropathy. Severe nephropathy manifested by azotemia, hematuria, and renal failure may occur in patients receiving methotrexate; fatalities have been reported. In one study, postmortem examination revealed extensive necrosis of the epithelium of the convoluted tubules. In patients with renal impairment, methotrexate accumulation and increased toxicity or additional renal damage may occur. For more Drug Warnings (Complete) data for METHOTREXATE (22 total), please visit the HSDB record page. Pharmacodynamics Methotrexate inhibits enzymes responsible for nucleotide synthesis which prevents cell division and leads to anti-inflammatory actions. It has a long duration of action and is generally given to patients once weekly. Methotrexate has a narrow therapeutic index. Do not take methotrexate daily. Methotrexate was originally developed as an anticancer drug but is now a first-line disease-modifying antirheumatic drug (DMARD) for RA. Its anti-arthritic mechanism is not only related to inhibiting folate-dependent enzymes but also to immunomodulatory effects (e.g., suppressing T cell activation, reducing pro-inflammatory cytokines) [1] - Methotrexate is effective in reducing RA symptoms, slowing joint destruction, and improving physical function in patients. It is often used as monotherapy or in combination with other DMARDs (e.g., TNF inhibitors) for moderate-to-severe RA [2] - L-carnitine can alleviate Methotrexate-induced large intestine toxicity in rats by reducing oxidative stress (decreasing MDA levels) and inhibiting inflammatory responses (lowering MPO activity), which may be related to its role in maintaining mitochondrial function and reducing lipid peroxidation [3] - The combination of Methotrexate and curcumin has a synergistic anti-arthritic effect in AIA rats, as it more effectively reduces joint inflammation and improves hematological indices compared to Methotrexate alone. Curcumin may also mitigate the mild hematological toxicity of Methotrexate by enhancing its anti-inflammatory activity and reducing oxidative stress [4] |
| 分子式 |
C20H22N8O5
|
|---|---|
| 分子量 |
454.44
|
| 精确质量 |
454.171
|
| 元素分析 |
C, 52.86; H, 4.88; N, 24.66; O, 17.60
|
| CAS号 |
59-05-2
|
| 相关CAS号 |
Methotrexate disodium;7413-34-5;Methotrexate hydrate;133073-73-1;Methotrexate monohydrate;6745-93-3; Methotrexate-d3; 432545-63-6; 7413-34-5 (disodium); 7532-09-4 (monosodium); 15475-56-6 (sodium); 59-05-2 (free acid)
|
| PubChem CID |
126941
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| 密度 |
1.4080
|
| 沸点 |
561.26°C
|
| 熔点 |
195°C
|
| 闪点 |
11℃
|
| 折射率 |
1.6910
|
| LogP |
-0.24
|
| tPSA |
210.54
|
| 氢键供体(HBD)数目 |
5
|
| 氢键受体(HBA)数目 |
12
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
33
|
| 分子复杂度/Complexity |
704
|
| 定义原子立体中心数目 |
1
|
| SMILES |
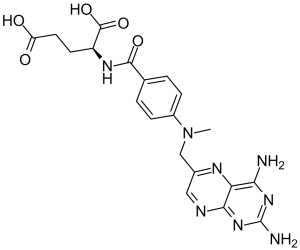
CN(CC1=CN=C2C(=N1)C(=NC(=N2)N)N)C3=CC=C(C=C3)C(=O)N[C@@H](CCC(=O)O)C(=O)O
|
| InChi Key |
FBOZXECLQNJBKD-ZDUSSCGKSA-N
|
| InChi Code |
InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1
|
| 化学名 |
(S)-2-(4-(((2,4-diaminopteridin-6-yl)methyl)(methyl)amino)benzamido)pentanedioic acid.
|
| 别名 |
alphamethopterin; amethopterin; methylaminopterin; CL 14377; NCIC04671; WR19039; WR-19039; Rheumatrex; Metatrexan; Hdmtx; Abitrexate; WR 19039; MTX; NCI-C04671; NCI C04671; CL14377; CL-14377; Methotrexate.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.50 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.50 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.50 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.5 mg/mL (5.50 mM) (饱和度未知) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 5 中的溶解度: 2% DMSO+30% PEG 300+5% Tween 80+ddH2O:5 mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2005 mL | 11.0026 mL | 22.0051 mL | |
| 5 mM | 0.4401 mL | 2.2005 mL | 4.4010 mL | |
| 10 mM | 0.2201 mL | 1.1003 mL | 2.2005 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT06123403 | Not yet recruiting | Diagnostic Test: blood methotrexate level and Cystatin C level |
Methotrexate Toxicity | Sohag University | January 2024 | |
| NCT06108453 | Enrolling by invitation | Drug: Methotrexate Sodium Drug: Rifampicin |
Drug Interactions | Seoul National University Bundang Hospital |
August 21, 2023 | Phase 1 |
| NCT03757364 | Completed | Drug: Methotrexate | Nail Psoriasis | Ryszard Górecki | January 7, 2018 | Ryszard Górecki |
| NCT04483466 | Enrolling by invitation | Drug: Methotrexate Drug: Placebo |
Investigate the Effect(s) of Methotrexate Treatment on Arthritis Disease Severity |
George Washington University | July 18, 2023 | Phase 3 |
|
|
|