| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
µM 米力农可将缺氧肌细胞中的 PKA 活性提高至含氧量正常水平。通过恢复 PKA 介导的调节性 TP 受体磷酸化,米力农 (50 nM) 可恢复缺氧肌细胞中 TP 受体的敏感性[1]。米力农通过显着减少 NE 引起的血管收缩来减弱最大张力的产生和 NE 敏感性。电压门控或 ATP 敏感 K+ 通道抑制不能阻止米力农诱导的 NE 反应减弱[4]。
|
||
|---|---|---|---|
| 体内研究 (In Vivo) |
在患有充血性心力衰竭 (CHF) 的大鼠中,米力农(1 μg/kg/min,静脉注射)可显着降低 PAP、PVR (-18.96 ± 1.7%) 和 LAP (-26.03 ± 2.3%)。吸入 1 mg/mL 米力农可将 PAP 几乎降低至接近最大值,但对 AP 没有实质性影响。更多的慢性心力衰竭大鼠组也经历了类似的肺动脉压力下降。在两组中,吸入米力农选择性地增加血浆中 cAMP 的含量,但不会增加 cGMP 的含量。随着时间的推移,重复吸入米力农甚至会降低肺湿重与干重的比率[2]。在中等左心室容积(0.08 mL/g 心肌)下,米力农 (49.5 µg) 显着提高收缩压-容积面积 (PVA(0.08)) 和收缩末压 (ESP(0.08))。它还使 ESPVR 向上移动。此外,米力农会适度降低 Ea 和 LV ESP(ESV)[3]。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
When administered as an IV bolus dose of 10-100 μg/kg, milrinone induces hemodynamic effects within 60 seconds reaching a peak effect by 2-5 minutes. The plasma AUC is significantly dose-dependent. Milrinone is primarily excreted in the urine, with 60% of a dose recovered after two hours and 90% within eight hours. Approximately 83% of milrinone recovered in urine is unchanged while 12% is present as the main O-glucuronide metabolite. Milrinone administered intravenously to congestive heart failure patients had a volume of distribution of 0.38 L/kg (injections between 12.5-125 μg/kg) and 0.45 L/kg (infusions between 0.2-0.7 μg/kg/min. Milrinone administered intravenously to congestive heart failure patients had a clearance of 0.13 L/kg/hr (injections between 12.5-125 μg/kg) and 0.14 L/kg/hr (infusions between 0.2-0.7 μg/kg/min. Metabolism / Metabolites Animal studies suggest that two oxidative pathways are involved in milrinone metabolism, albeit only involving a small proportion of the administered dose. The major metabolite is the O-glucuronide metabolite. Biological Half-Life Milrinone administered intravenously to congestive heart failure patients had a mean terminal elimination half-life of 2.3 hours (injections between 12.5-125 μg/kg) and 2.4 hours (infusions between 0.2-0.7 μg/kg/min. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
Milrinone is approximately 70% bound to human plasma proteins. |
||
| 参考文献 |
|
||
| 其他信息 |
Pharmacodynamics
Milrinone is a bipyridine derivative with positive inotropic and lusitropic effects that also results in peripheral vasodilation with minimal chronotropic effects over a therapeutic range of 100 to 300 ng/mL. As such, milrinone is used in decompensated congestive heart failure. Studies have demonstrated that milrinone exhibits sigmoidal effects, such that increasing milrinone plasma concentrations beyond a certain level results in no further hemodynamic changes. Despite milrinone's benefits, both intravenous and oral use has been associated with increased frequency of ventricular arrhythmias, and long-term oral use has been associated with an increased risk of sudden death; in general, there are no data to support the safety or efficacy of milrinone use beyond 48 hours and patients should be monitored closely for cardiac dysfunction. Also, as milrinone is primarily excreted renally, dose adjustments may be required in patients with impaired renal function. |
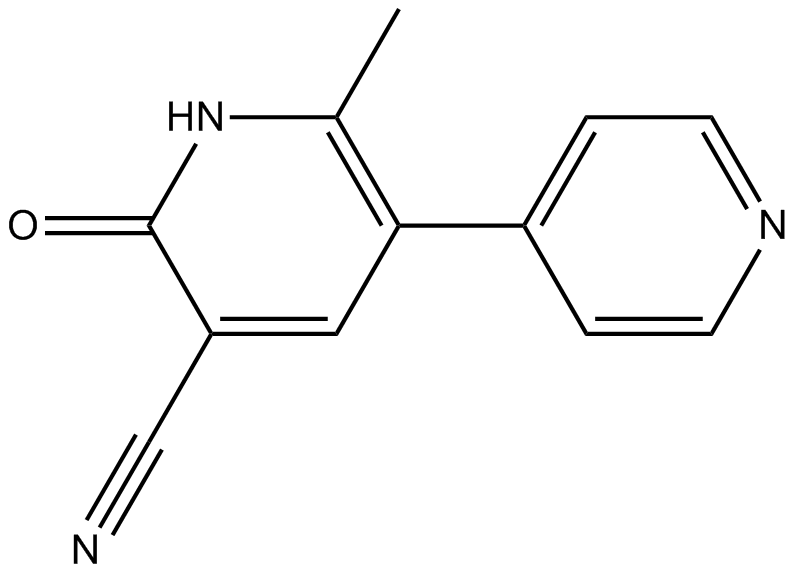
| 分子式 |
C12H9N3O
|
|
|---|---|---|
| 分子量 |
211.22
|
|
| 精确质量 |
211.074
|
|
| CAS号 |
78415-72-2
|
|
| 相关CAS号 |
Milrinone lactate;100286-97-3;Milrinone-d3;2749393-50-6
|
|
| PubChem CID |
4197
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
448.7±45.0 °C at 760 mmHg
|
|
| 熔点 |
>3000C
|
|
| 闪点 |
225.2±28.7 °C
|
|
| 蒸汽压 |
0.0±1.1 mmHg at 25°C
|
|
| 折射率 |
1.622
|
|
| LogP |
0.41
|
|
| tPSA |
69.54
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
3
|
|
| 可旋转键数目(RBC) |
1
|
|
| 重原子数目 |
16
|
|
| 分子复杂度/Complexity |
419
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
PZRHRDRVRGEVNW-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C12H9N3O/c1-8-11(9-2-4-14-5-3-9)6-10(7-13)12(16)15-8/h2-6H,1H3,(H,15,16)
|
|
| 化学名 |
6-methyl-2-oxo-5-pyridin-4-yl-1H-pyridine-3-carbonitrile
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.75 mg/mL (13.02 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 27.5 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.75 mg/mL (13.02 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 27.5 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.7344 mL | 23.6720 mL | 47.3440 mL | |
| 5 mM | 0.9469 mL | 4.7344 mL | 9.4688 mL | |
| 10 mM | 0.4734 mL | 2.3672 mL | 4.7344 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05122884 | Recruiting | Drug: Milrinone | Septic Shock Cardiac Output |
Mahidol University | December 1, 2021 | Phase 2 |
| NCT06077721 | Recruiting | Drug: Milrinone | Ischemic Heart Disease Valvular Heart Disease |
Konkuk University Medical Center | November 3, 2023 | |
| NCT04484675 | Recruiting | Drug: Milrinone inhalation Drug: Milrinone infusion |
Pulmonary Hypertension Due to Left Heart Disease |
Zagazig University | January 20, 2022 | Phase 4 |
| NCT04362527 | Recruiting | Drug: Milrinone 1 Mg/mL Solution for Injection |
Vasospasm | University Hospital, Angers | August 10, 2020 | Phase 3 |
|
|---|
|
|