| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Akt1 (IC50 = 8 nM); Akt2 (IC50 = 12 nM); Akt3 (IC50 = 65 nM)
|
|---|---|
| 体外研究 (In Vitro) |
MK-2206 是一种变构抑制剂,由 pleckstrin 同源结构域激活。 MK-2206 可防止 Akt 在 T308 和 S473 处的自磷酸化。此外,MK-2206 还可阻断 Akt 介导的下游信号分子(如 TSC2、PRAS40 和核糖体 S6 蛋白)的磷酸化。 [1] MK-2206 比 Ras 突变细胞系(NCI-H358、NCI-H23、NCI-H1299 和 Calu-)更有效地抑制 Ras 野生型 (WT) 细胞系(A431、HCC827 和 NCI-H292)。 6).在肺 NCI-H460 或卵巢 A2780 肿瘤细胞中,MK-2206 与厄洛替尼或拉帕替尼等细胞毒性药物联合使用时也表现出协同反应。 [2] MK-2206 或 siRNA 介导的 Akt 抑制可强烈诱导人神经胶质瘤细胞发生自噬。然而,真核延伸因子 2 (eEF-2) 沉默会抑制 MK-2206 诱导的自噬,同时促进细胞凋亡。 [3]
|
| 体内研究 (In Vivo) |
MK-2206 在 240 mg/kg 剂量下显示 60% TGI,并抑制 A2780 卵巢癌异种移植物中超过 70% 的磷酸-Akt1/2(T308 和 S473)。在 NCI-H292 异种移植物中,MK-2206 与厄洛替尼或拉帕替尼联合使用时表现出显着的抗肿瘤活性。 [2]
在携带A2780卵巢癌症异种移植物的裸鼠中,单次口服剂量为240 mg/kg的MK-2206导致肿瘤中磷酸化-Akt1/2(T308和S473)的持续抑制(>70%)。在相同的肿瘤模型中,MK-2206通过8776抑制肿瘤生长;当以240mg/kg每天口服一周三次时,60%。我们进一步评估了MK-2206与化疗药物或受体酪氨酸激酶抑制剂联合使用的效果。当MK-2206与具有不同作用模式的细胞毒性药物联合使用时,可以看到相加或协同作用,包括拓扑异构酶抑制剂(阿霉素和喜树碱)、抗代谢物(吉西他滨和5-FU)、抗微管(多西他赛)和DNA交联剂(卡铂)。Akt抑制致敏这些药物诱导肿瘤细胞凋亡。在体内,MK-2206增强了多西他赛、吉西他滨和卡铂在裸鼠异种移植物肿瘤模型中的抗肿瘤效果。当MK-2206与EGFR抑制剂埃洛替尼在非小细胞肺癌癌症细胞系中联合或与EGFR-Her2双重抑制剂拉帕替尼在乳腺癌症细胞系中组合时,也观察到体外协同相互作用。MK-2206的共治疗增强了埃洛替尼或拉帕替尼的抗肿瘤活性,并导致肺癌和乳腺癌症模型中的肿瘤消退。研究了MK-2206与这些药物之间协同作用的生化机制。MK-2206在临床前研究中通常具有良好的耐受性。在用MK-2206治疗的动物中,观察到血糖和胰岛素的机制相关药效学变化。高血糖和高胰岛素血症都是轻度和短暂的,在治疗结束后恢复到基线水平。这些临床前结果支持MK-2206在人类中的进一步临床开发。[1] |
| 酶活实验 |
Akt 激酶通过 GSK 衍生的生物素化肽底物进行检测。通过将磷酸肽特异的镧系螯合物 (Lance) 偶联单克隆抗体与链霉亲和素连接的别藻蓝蛋白 (SA-APC) 荧光团(可与肽的生物素部分结合)相结合,可以使用均相时间分辨荧光 (HTRF)以确定磷酸化程度。当喷枪和APC靠近时,喷枪将非辐射能量转移到APC,然后APC发射波长为655 nm的光。蛋白酶抑制剂混合物 (PIC) 100X:苯甲脒 1 mg/mL、胃酶抑素 0.5 mg/mL、亮肽素 0.5 mg/mL、抑肽酶 0.5 mg/mL; 10X 检测试剂:20 mM 9-甘油磷酸、50 mM HEPES、pH 7.3、16.6 mM EDTA、0.1% BSA、0.1% Triton X-100、0.17 nM 标记单克隆抗体和 0.0067 mg/mL SA-APC 组成淬灭缓冲液。 ATP/MgCl2 测定的工作溶液:1X 测定缓冲液、1 mM DTT、1X PIC、5% 甘油、活性 Akt;肽工作溶液:2 TM GSK 生物素化肽、1X 检测缓冲液、1 mM DTT、1X PIC 和 5% 甘油。通过向适当的孔中添加 16 µL ATP/MgCl2 工作溶液来组装反应。添加 MK-2206 或载体 (1.0 µL),然后添加 10 µL 肽工作溶液。加入 13 μL 酶工作液并混合开始反应。允许反应进行 50 分钟,然后通过添加 60 µL HTRF 淬灭缓冲液来停止。停止的反应在室温下孵育至少30分钟,然后在仪器中读数。
|
| 细胞实验 |
在 96 孔板中,接种 2-3 × 103 个细胞,然后将板孵育 24 小时。之后,向细胞添加 MK-2206(0、0.3、1 和 3 μM)。 72或96小时后,评估细胞增殖。
细胞增殖试验和组合指数测定[2] 在96孔板中以每孔2至3×103的密度接种细胞。镀敷后24小时,将不同浓度的药物(作为单一试剂或组合)加入孔中。在给药后72或96小时使用CellTiter-Glo测定法测定细胞增殖。根据Chou和Talalay的方法,使用组合指数(CI)评估药物相互作用的性质。从Calcusyn获得了一个商业软件包。在与多西他赛的组合中,我们测试了三种治疗序列:(a)MK-2206,然后是多西他赛——细胞暴露于MK-2206 24小时,然后在MK-2206洗脱后,细胞用多西他赛再治疗72小时;(b) 多西他赛,然后是MK-2206——细胞暴露于多西他赛24小时,然后在多西他赛洗脱后,细胞用MK-2206再处理72小时;以及(c)同时治疗——细胞暴露于MK-2206和多西他赛72小时。[2] 在添加10%胎牛血清的培养基中培养的LN229和T98G细胞用一系列浓度的MK-2206处理,并通过Western blot检测磷酸化-eEF-2和eEF-2的水平。使用微管蛋白作为负荷控制。(B) 用非靶向RNA或靶向eEF-2激酶的siRNA转染LN229和T98 G细胞,然后用MK-2206处理24小时。Western检测eEF-2激酶、LC3和p62。使用微管蛋白作为负荷控制。[3] MK-2206对人脑胶质瘤细胞自噬的影响[3] (A) 在添加10%胎牛血清的培养基中培养的LN229和T98G细胞用MK2206处理24小时,通过Western blot检测LC3水平。(B) 在10nM巴非霉素A1存在或不存在的情况下,用MK2206处理LN229和T98G细胞24小时,并通过Western blot检测LC3水平。使用微管蛋白作为负荷控制。(C) LN229和T98G细胞用GFP-LC3质粒转染,然后用2.5或5μM MK2206处理24小时。治疗结束时,在60倍放大镜下检查细胞中GFP-LC3斑点的数量。条形图是具有10个或更多GFP-LC3斑点的细胞百分比的量化。每次治疗中至少对100个细胞进行评分。*p<0.05;**p<0.01,t检验,MK-2206与赋形剂相比。(D) 用10μM MK-2206处理LN229和T98G细胞24小时,用流式细胞术分析处理细胞中的AO荧光强度。(E) 用MK-2206(2.5μM)或载体处理的LN229细胞通过胰蛋白酶消化收获,固定并包埋在刺树脂中。切割90nm薄切片,用JEOL 1200EX透射电子显微镜在80Kv下检查。箭头表示自噬空泡。 |
| 动物实验 |
SK-OV-3, NCI-H292, HCC70, PC-3, and NCI-H460 models in male CD1-nude mice
120 mg/kg Orally administered Efficacy studies in mouse xenograft models[2] Human tumor cells were suspended in 50% Matrigel (BD) and 50% PBS and were injected s.c. into the left flank of the mice.[2] When the mean tumor size reached 0.13 cm3 for the SK-OV-3 or 0.2 cm3 for the NCI-H292, HCC70, PC-3, and NCI-H460 models, the mice were randomized into control and treatment groups with approximately equivalent ranges of tumor volume between groups (n = 5 animals per group). The following vehicles were used to dose the compounds: 30% Captisol for MK-2206; 0.5% methylcellulose + 0.1% Tween 80 for erlotinib; distilled water for lapatinib; 0.73% ethanol in saline for docetaxel; and saline for carboplatin and gemcitabine. The control group received vehicle only. Tumor volume was measured with calipers twice a week. Animal body weight and physical signs were monitored during the experiments. Briefly, 4–6 week-old female nude mice were inoculated subcutaneously with LN229 cells (5 × 106 cells/per site) with or without silencing of eEF-2 kinase. At day 7 after inoculation, MK-2206 (120 mg/kg, p.o.) was administered to the tumor-bearing mice. Tumors were harvested 24 h post drug administration for analysis of autophagy and apoptosis. Apoptosis was measured using the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling tetramethylrhodamine red apoptosis kit from Roche, and using Western blot analysis of cleaved caspase 3. Autophagy was detected by Western blot analysis of LC3 II.[3] |
| 参考文献 | |
| 其他信息 |
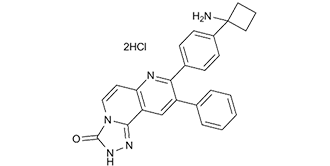
MK-2206 is an organic heterotricyclic compound that is [1,2,4]triazolo[3,4-f][1,6]naphthyridin-3(2H)-one substituted at positions 8 and 9 respectively by 4-(1-aminocyclobutyl)phenyl and phenyl groups. It has a role as an EC 2.7.1.137 (phosphatidylinositol 3-kinase) inhibitor. It is functionally related to a 1,6-naphthyridine.
Akt Inhibitor MK2206 is an orally bioavailable allosteric inhibitor of the serine/threonine protein kinase Akt (protein kinase B) with potential antineoplastic activity. Akt inhibitor MK2206 binds to and inhibits the activity of Akt in a non-ATP competitive manner, which may result in the inhibition of the PI3K/Akt signaling pathway and tumor cell proliferation and the induction of tumor cell apoptosis. Activation of the PI3K/Akt signaling pathway is frequently associated with tumorigenesis and dysregulated PI3K/Akt signaling may contribute to tumor resistance to a variety of antineoplastic agents. MK-2206 is currently being studies in two Phase I trials, one in healthy volunteers (HV) and one in cancer patients. In the first-in-human HV trial, twenty-four healthy, male subjects participated in this Phase I randomized, double-blind, placebo-controlled, sequential-panel, multiple-period, rising single oral dose study. Eight (8) subjects were assigned to each of 3 panels (Panels A, B, and C) where in each treatment period in a panel the same 6 subjects received MK-2206 and 2 subjects received placebo after an overnight fast. The volunteers were administered single doses from 0.25 to 100 mg and blood samples were collected predose and at prespecified postdose time points for pharmacokinetic and pharmacodynamic (whole blood inhibition of phospho Akt) assays. Single doses of MK-2206, up to 100 mg, were found to be generally well tolerated. No serious clinical or laboratory adverse experience was reported. The most commonly reported adverse experiences were headache, common cold, and diarrhea. One subject was discontinued from the study due to the clinical adverse experience of blurry vision which resolved. There were no clinically meaningful changes in laboratory safety tests or ECG evaluations. No clinically significant hyperglycemia or hyperinsulinemia was seen in these subjects. Preliminary pharmacokinetic results found that orally administered MK-2206 was readily absorbed with a median Tmax of 6 to 8 hours. The median half-life was 55 to 78 hours. AUC0-\#8734; and Cmax displayed dose proportional behavior from 2-mg to 100-mg. Preliminary pharmacodynamic results found that single doses of 40-, 80- and 100-mg MK-2206 inhibited Akt in whole blood to a greater extent than placebo. Maximum Akt inhibition occurred at 6 hours postdose for both the 80- and 100-mg doses with mean plasma concentrations of >65 nM. There was evidence of Akt inhibition from 2 through 24 hours. In conclusion, MK-2206 was generally well tolerated following single dose administration to healthy subjects. MK-2206 displays dose proportional pharmacokinetics with clear evidence of Akt inhibition. Clinical development of MK-2206 in cancer patients is ongoing with a focus on tumors harboring PI3K pathway activation events.[1] The serine/threonine kinase Akt lies at a critical signaling node downstream of phosphatidylinositol-3-kinase and is important in promoting cell survival and inhibiting apoptosis. An Akt inhibitor may be particularly useful for cancers in which increased Akt signaling is associated with reduced sensitivity to cytotoxic agents or receptor tyrosine kinase inhibitors. We evaluated the effect of a novel allosteric Akt inhibitor, MK-2206, in combination with several anticancer agents. In vitro, MK-2206 synergistically inhibited cell proliferation of human cancer cell lines in combination with molecular targeted agents such as erlotinib (an epidermal growth factor receptor inhibitor) or lapatinib (a dual epidermal growth factor receptor/human epidermal growth factor receptor 2 inhibitor). Complementary inhibition of erlotinib-insensitive Akt phosphorylation by MK-2206 was one mechanism of synergism, and a synergistic effect was found even in erlotinib-insensitive cell lines. MK-2206 also showed synergistic responses in combination with cytotoxic agents such as topoisomerase inhibitors (doxorubicin, camptothecin), antimetabolites (gemcitabine, 5-fluorouracil), anti-microtubule agents (docetaxel), and DNA cross-linkers (carboplatin) in lung NCI-H460 or ovarian A2780 tumor cells. The synergy with docetaxel depended on the treatment sequence; a schedule of MK-2206 dosed before docetaxel was not effective. MK-2206 suppressed the Akt phosphorylation that is induced by carboplatin and gemcitabine. In vivo, MK-2206 in combination with these agents exerted significantly more potent tumor inhibitory activities than each agent in the monotherapy setting. These findings suggest that Akt inhibition may augment the efficacy of existing cancer therapeutics; thus, MK-2206 is a promising agent to treat cancer patients who receive these cytotoxic and/or molecular targeted agents.[2] Inhibition of the survival kinase Akt can trigger apoptosis, and also has been found to activate autophagy, which may confound tumor attack. In this study, we investigated regulatory mechanisms through which apoptosis and autophagy were modulated in tumor cells subjected to Akt inhibition by MK-2206, the first allosteric small molecule inhibitor of Akt to enter clinical development. In human glioma cells, Akt inhibition by MK-2206 or siRNA-mediated attenuation strongly activated autophagy, whereas silencing of eukaryotic elongation factor-2 (eEF-2) kinase, a protein synthesis regulator, blunted this autophagic response. Suppression of MK-2206-induced autophagy by eEF-2 silencing was accompanied by a promotion of apoptotic cell death. Similarly, siRNA-mediated inhibition of eEF-2 kinase potentiated the efficacy of MK-2206 against glioma cells. Together, these results showed that blunting autophagy and augmenting apoptosis by inhibition of eEF-2 kinase could modulate the sensitivity of glioma cells to Akt inhibition. Our findings suggest that targeting eEF-2 kinase may reinforce the antitumor efficacy of Akt inhibitors such as MK-2206.[3] |
| 分子式 |
C25H23CL2N5O
|
|---|---|
| 分子量 |
480.39
|
| 精确质量 |
479.127
|
| 元素分析 |
C, 62.24; H, 5.22; Cl, 14.70; N, 14.52; O, 3.32
|
| CAS号 |
1032350-13-2
|
| 相关CAS号 |
MK-2206 free base;1032349-93-1;MK-2206;1032349-77-1
|
| PubChem CID |
24964624
|
| 外观&性状 |
Yellow solid powder
|
| LogP |
6.547
|
| tPSA |
89.07
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
31
|
| 分子复杂度/Complexity |
760
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=C1N([H])N=C2C3=C([H])C(C4C([H])=C([H])C([H])=C([H])C=4[H])=C(C4C([H])=C([H])C(=C([H])C=4[H])C4(C([H])([H])C([H])([H])C4([H])[H])N([H])[H])N=C3C([H])=C([H])N21
|
| InChi Key |
HWUHTJIKQZZBRA-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C25H21N5O.2ClH/c26-25(12-4-13-25)18-9-7-17(8-10-18)22-19(16-5-2-1-3-6-16)15-20-21(27-22)11-14-30-23(20)28-29-24(30)31;;/h1-3,5-11,14-15H,4,12-13,26H2,(H,29,31);2*1H
|
| 化学名 |
8-[4-(1-aminocyclobutyl)phenyl]-9-phenyl-2H-[1,2,4]triazolo[3,4-f][1,6]naphthyridin-3-one;dihydrochloride
|
| 别名 |
MK2206 HCl; MK2206 dihydrochloride; MK2206; MK-2206 dihydrochloride; MK-2206 2HCl; MK2206; 8-(4-(1-Aminocyclobutyl)phenyl)-9-phenyl-[1,2,4]triazolo[3,4-f][1,6]naphthyridin-3(2H)-one dihydrochloride; 8-[4-(1-AMINOCYCLOBUTYL)PHENYL]-9-PHENYL-1,2,4-TRIAZOLO[3,4-F][1,6]NAPHTHYRIDIN-3(2H)-ONE DIHYDROCHLORIDE; Q34I3E28IO; 8-[4-(1-aminocyclobutyl)phenyl]-9-phenyl-2H-[1,2,4]triazolo[3,4-f][1,6]naphthyridin-3-one;dihydrochloride; MK 2206; MK-2206
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~14 mg/mL (~29.1 mM)
Water: <1 mg/mL Ethanol: <1 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1.67 mg/mL (3.48 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 16.7 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 1.67 mg/mL (3.48 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 16.7mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: 15% Captisol: 17mg/mL 配方 4 中的溶解度: 25 mg/mL (52.04 mM) in 20% SBE-β-CD in Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0816 mL | 10.4082 mL | 20.8164 mL | |
| 5 mM | 0.4163 mL | 2.0816 mL | 4.1633 mL | |
| 10 mM | 0.2082 mL | 1.0408 mL | 2.0816 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Growth-inhibitory effect of MK-2206 on nasopharyngeal carcinoma cell lines.Drug Des Devel Ther. 2014 Oct 10;8:1827-37. |
MK-2206 induces cell cycle arrest at G1 in a dose-dependent manner in CNE-2 and HONE-1 cells.Drug Des Devel Ther. 2014 Oct 10;8:1827-37. |
No apoptosis was induced by MK-2206 in the four nasopharyngeal carcinoma cell lines.Drug Des Devel Ther. 2014 Oct 10;8:1827-37. |
MK-2206 inhibited phosphorylation of AKT downstream targets.SUNE-1 and CNE-2 cells were treated with different concentrations of MK-2206 for 24 hours.Drug Des Devel Ther. 2014 Oct 10;8:1827-37. |
Effect of MK-2206 on autophagy in human nasopharyngeal carcinoma cells.Drug Des Devel Ther. 2014 Oct 10;8:1827-37. |
Effects of MK-2206 on tumor growth of human CNE-2 xenografts in nude mice.Drug Des Devel Ther. 2014 Oct 10;8:1827-37. |
|
|
|