| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |

| 靶点 |
Tubulin; microtubule
|
|---|---|
| 体外研究 (In Vitro) |
MMAF 可预防间变性大细胞淋巴瘤的生长。体外细胞毒性测定对 Karpas 299、乳腺癌 H3396、肾细胞癌 786-O 和 Caki-1 细胞的 IC50 值分别为 119、105、257 和 200 nM[4]。
体外生长抑制。cAC10−L1−MMAF4在CD30阳性和阴性细胞系上进行了测试,如表2所示。将细胞连续暴露于ADC 96小时,并将Alamar Blue转化率测定的细胞毒性作用与游离MMAF进行比较。以摩尔计,cAC10−L1−MMAF4的效力平均比游离的MMAF高2200倍以上,并且对所有测试的CD30阳性细胞系都有活性。这些作用是免疫特异性的,因为不表达可检测水平的CD30抗原的WSU-NHL细胞不受偶联物的影响。还对基于cAC10−L1−MMAF-OMe4的偶联物进行了有限的研究,发现它们对Karpas 299(0.04 nM)和L540cy(0.11 nM)细胞的IC50值与cAC10-L1-MMAF4的IC50值相当(表2)。这与之前所述的假设一致,即MMAF OMe的活性形式是游离酸MMAF[4]。 对LewisY阳性人小细胞肺癌细胞系(H69)及其P-糖蛋白过表达的对应物H69/LX4进行了进一步的研究,使用MMAF和cBR96-L1-MMAF8缀合物。将结果与阿霉素和cBR96-L1−doxorbicin8进行了比较,这是一种之前描述的与8个阿霉素分子/mAb的偶联物。与亲本细胞相比,H69/LX-4细胞对阿霉素的敏感性低约100倍(图2A),而与MMAF的差异仅为3倍(图2B)。这一趋势延伸到偶联物,因为耐药细胞系对BR96-L1-doxorbicin8耐药(图2C),但对相应的MMAF偶联物非常敏感(图2D)。这些结果已在几种MDR阳性细胞系上得到证实(数据未显示),其中发现MMAF和基于MMAF的免疫偶联物绕过了常见形式的MDR表型[4]。 为了评估结合亲和力对RDCs细胞毒性活性的影响,我们使用对EGFR具有不同结合亲和力的repebody合成了三种不同的药物偶联物,并检测了它们对EGFR过表达HCC827细胞的细胞毒性(图S9和表S1)。与其他两种低亲和力偶联物相比,rEgH9-MMAF偶联物表现出最高的细胞毒性。这一结果表明,repebody对EGFR的结合亲和力对RDCs的细胞毒性活性至关重要,优化结合亲和力可以增强药物偶联物的细胞毒性。为了研究EGFR的细胞表面表达水平与RDCs的细胞毒性之间的关系,我们培养了三种表达不同EGFR水平的癌症细胞系(图 3 a) 用不同浓度的rEgH9-MMAF偶联物处理三天(图3b-d)。repebody-MMAF偶联物以剂量依赖的方式对A431和HCC827细胞显示出强烈的细胞毒性作用,导致有效半最大浓度(EC50)分别为1.4 nM和0.072 nM(表1)。这一结果表明,repebody-MMAF缀合物对HCC827细胞的细胞毒性远高于游离、细胞不可渗透的MMAF(EC50=117.9 nM)和裸repebodys(rEgH9:EC50=17.2 nM)。有趣的是,repebody-MMAF偶联物在HCC827细胞中的效力远高于A431细胞。这一结果似乎是由于HCC827细胞表达组成型内化的致癌EGFR,因此对repebody-MMAF偶联物的摄取增加。15即使在高剂量下(>200 nM;表 1). 我们的研究结果表明,RDCs可以以受体特异性的方式有效地将强效抗癌药物递送到靶细胞,最大限度地减少脱靶效应。上得到证实(数据未显示),其中发现MMAF和基于MMAF的免疫偶联物绕过了常见形式的MDR表型[4]。 |
| 体内研究 (In Vivo) |
与MMAE(1 mg/kg)相比,小鼠对MMAF的最大耐受剂量(MTD)明显更大(>16 mg/kg)。与 cAC10-L1-MMAF4 在小鼠中的 50 mg/kg MTD 相比,等效的 cAC10-L4-MMAF4 ADC 危险性较小,关联和相关 MTD 分别 >150 mg/kg 和 90 mg/kg。 [4]。
体内治疗研究。[4] 在患有皮下或播散性Karpas 299肿瘤的裸鼠中测定ADC的治疗效果。在患有皮下肿瘤的动物中,单次注射cAC10-L1-MMAF4或cAC10-L4-MMAF4可获得显著的抗肿瘤作用,其效力和活性难以区分(图5A)。几乎所有接受2mg抗体成分/kg体重单次ADC注射的动物都治愈了。当剂量降至1mg/kg时,两种ADC的疗效以明显相等的方式下降。在这个剂量下,分别用cAC10-L1-MMAF4和cAC10-L4-MMAF4 ADC治愈的六只动物中仍有两只和三只。 免疫组织化学分析。[4] 进行了研究,以确定cAC10−L1−MMAF4是否定位在裸鼠皮下Karpas 299肿瘤中。动物静脉注射cAC10-L1-MMAF4或cBR96-L1-MMAF4,每公斤体重10毫克抗体成分。24小时后切除肿瘤,分别使用生物素化的抗人Fc和抗MMAF单克隆抗体作为二抗,使用免疫组织化学评估冷冻切片中是否存在单克隆抗体和药物成分。图4A和4C显示,与cBR96非结合对照ADC相比,cAC10−L1−MMAF4在卡帕斯299肿瘤中的积累效率更高(图4B和4D)。由于单克隆抗体(图4A)和药物(图4C)部分在整个肿瘤中都被检测到,我们得出结论,ADC是作为一个完整的分子递送的。 研究人员还使用HCC827细胞评估了rEgH9-MMAF偶联物在异种移植物小鼠中的抗肿瘤活性。当肿瘤体积达到110至130 mm3时,小鼠每天静脉注射(10 mg kg-1)repebody-MMAF偶联物或裸repebody(rEgH9)六天(图4a)。作为阳性对照,使用西妥昔单抗。repebody-MMAF偶联物显示出显著的肿瘤消退反应(第二天残留肿瘤33.4%) 20, ***P<0.001)与裸瘤(第20天残留肿瘤83.4%,P> 0.05)和西妥昔单抗。在第20天,除了短暂的体重减轻外,未在接受治疗的小鼠中检测到明显的不良反应(P>0.05)(图4b)。 |
| 酶活实验 |
Released Drug Identification. [4]
L540cy细胞的溶酶体提取物是通过将2.4×108个细胞在9 mL 0.25 M蔗糖、1 mM EDTA和10 mM HEPES(4-(2-羟乙基)哌嗪-1-乙磺酸)(pH 7.4)中溶胀而制备的。在冰上放置30分钟后,将细胞进行Dounce均质化,直至通过台盼蓝染料排斥法测得>95%的细胞破碎。将匀浆离心(3000g,10分钟,4°C)以沉淀细胞碎片,将上清液(每管4×107个细胞当量)转移到聚铝醇超离心管(13×51 mm)中,并在TLA100.3转子中离心(17000g,15分钟,4℃)以分离含溶酶体的轻线粒体沉淀。颗粒储存在-80°C下。将颗粒解冻并重新悬浮在500μL pH 5.0的50 mM乙酸钠和2 mM DTT中。将cAC10−L4-[12C]MMAF4、cAC10−于L4-[13C]MMAF4和cAC10−着L4-[12/13C]MMAF4(50μg/mL)独立添加到一个颗粒中。经过三次冻融循环以打开溶酶体后,样品在37°C下孵育24小时。加入冷甲醇(2体积)沉淀蛋白质,将样品在14000g下离心至颗粒碎片,如上所述通过低分辨率质谱分析100μL上清液。通过在室温下用1 mM半胱氨酸在PBS中处理100μM L4-MMAF 10分钟,制备了真正的半胱氨酸-L4-MMAF。 |
| 细胞实验 |
根据细胞系的不同,用连续稀释的测试分子处理细胞并孵育四到六天。 Alamar Blue 染料还原测定用于评估细胞生长并减少数据,以产生 IC50 值[2]。
体外生长抑制。[4] 收集细胞的对数相培养物,并根据预定条件以500至10000个细胞/孔的接种密度接种细胞。在孵育24小时以允许表面蛋白重构后,加入一系列稀释的测试分子,并根据细胞系将培养物再孵育4-6天。根据先前发表的方法,使用如前所述的Alamar Blue染料还原测定法对细胞生长和数据减少进行评估以产生IC50值。简而言之,在添加培养物之前,在完全培养基中新鲜制备了40%的Alamar Blue溶液(wt/vol)。在药物暴露92小时后,将Alamar Blue溶液加入细胞中以构成10%的培养体积。将细胞孵育4小时,并在Fusion HT荧光平板读数器上测量染料减少。 |
| 动物实验 |
Mice: The size of the subcutaneous Karpas 299 tumor is 300 mm3, and three animals per group are given one intravenous injection of either cAC10-L1-MMAF4 or cBR96-L1-MMAF4 (10 mg antibody component/kg body weight). Immunohistochemistry evaluation is used to stain 5 μm-thin frozen tissue sections after the tumors have been removed and placed in an optimal cutting temperature compound[1].
In Vivo Therapy Experiments. [4] For the localized, subcutaneous disease model of anaplastic large cell lymphoma, 5 × 106 Karpas 299 cells were implanted into the right flanks of C.B.-17 SCID mice. Therapy was initiated when the tumor size in each group of six animals averaged approximately 100 mm3. Treatments consisted of a single injection of solutions of the conjugates or controls in PBS intravenously (tail vein). Tumor volume was determined using the formula (L × W2)/2. For the disseminated ALCL model, 1 × 106 Karpas 299 were injected in the tail vein into C. B.-17 SCID mice. Single dose injection treatment was performed at 9 days after tumor injection. In Vivo Localization of Antibodies via Immunohistochemistry. [4] When subcutaneous Karpas 299 tumor size reached 300 mm3, three animals per group received one injection of 10 mg antibody component/kg body weight of either cAC10−L1−MMAF4 or cBR96−L1−MMAF4 intravenously. Tumors were then removed and placed in optimal cutting temperature (OCT) compound, and 5 μm-thin frozen tissue sections were stained using immunohistochemistry evaluation. Briefly, frozen tissues on the slides were air-dried then fixed in 4% paraformaldehyde for 15 min at room temperature. Endogenous peroxidase activity was blocked using 0.6% H2O2 for 15 min. Additional blocking for endogenous biotin was done using the Avidin−Biotin Blocking kit. Biotinylated-anti-human-Fc and biotinylated-anti-drug antibodies were incubated on tissues at 2 μg/mL concentration for 1 h at room temperature. Following incubation of slides with avidin conjugated to HRP, 3,3‘-diabenzidine (DAB) was used as a substrate for HRP. Tissues were counterstained using hematoxylin, slides were dehydrated, and slips were applied. Images were taken using the Zeiss Axiovert light microscope. In vivo antitumor activity of the RDCs.[2] a) Nude xenograft mice (HCC827) were administered with the rEgH9–MMAF conjugates, naked repebodies, or cetuximab (10 mg kg−1) intravenously every day for six days after tumor establishment. The tumor size was measured every third day for 20 days (mean±SD; n=6). b) Changes in the mouse body weight. After administration, the body weights of each mouse were measured every third day. |
| 毒性/毒理 (Toxicokinetics/TK) |
Maximum Tolerated Doses. [4]
The maximum tolerated doses (MTDs) of the cAC10−MMAF conjugates were determined in BALB/c mice and in Sprague−Dawley rats and are defined as the highest dose that did not induce >20% weight loss, distress, or overt toxicities in any of the animals. This dose was generally within 20% of doses where such events took place. cAC10−L1−MMAF4 has an MTD of 50 mg/kg in mice and 15 mg/kg in rats. The corresponding cAC10−L4−MMAF4 ADC was much less toxic, having MTDs in mice and rats of >150 mg/kg (the highest dose tested, which resulted in no apparent toxicity) and 90 mg/kg in rats, respectively. This clearly indicates that the method by which the drug is attached to the mAb can have a pronounced effect on ADC tolerability, and the ADC lacking the peptide spacer within the linker was much less toxic than the peptide-based ADC. |
| 参考文献 |
|
| 其他信息 |
Targeted therapy based on protein-drug conjugates has attracted significant attention owing to its high efficacy and low side effects. However, efficient and stable drug conjugation to a protein binder remains a challenge. Herein, a chemoenzymatic method to generate highly stable and homogenous drug conjugates with high efficiency is presented. The approach comprises the insertion of the CaaX sequence at the C-terminal end of the protein binder, prenylation using farnesyltransferase, and drug conjugation through an oxime ligation reaction. MMAF and an EGFR-specific repebody are used as the antitumor agent and protein binder, respectively. The method enables the precisely controlled synthesis of repebody-drug conjugates with high yield and homogeneity. The utility of this approach is illustrated by the notable stability of the repebody-drug conjugates in human plasma, negligible off-target effects, and a remarkable antitumor activity in vivo. The present method can be widely used for generating highly homogeneous and stable PDCs for targeted therapy.[2]
Antibody-drug conjugates utilize the antibody as a delivery vehicle for highly potent cytotoxic molecules with specificity for tumor-associated antigens for cancer therapy. Critical parameters that govern successful antibody-drug conjugate development for clinical use include the selection of the tumor target antigen, the antibody against the target, the cytotoxic molecule, the linker bridging the cytotoxic molecule and the antibody, and the conjugation chemistry used for the attachment of the cytotoxic molecule to the antibody. Advancements in these core antibody-drug conjugate technology are reflected by recent approval of Adectris(®) (anti-CD30-drug conjugate) and Kadcyla(®) (anti-HER2 drug conjugate). The potential approval of an anti-CD22 conjugate and promising new clinical data for anti-CD19 and anti-CD33 conjugates are additional advancements. Enrichment of antibody-drug conjugates with newly developed potent cytotoxic molecules and linkers are also in the pipeline for various tumor targets. However, the complexity of antibody-drug conjugate components, conjugation methods, and off-target toxicities still pose challenges for the strategic design of antibody-drug conjugates to achieve their fullest therapeutic potential. This review will discuss the emergence of clinical antibody-drug conjugates, current trends in optimization strategies, and recent study results for antibody-drug conjugates that have incorporated the latest optimization strategies. Future challenges and perspectives toward making antibody-drug conjugates more amendable for broader disease indications are also discussed. [3] We have previously shown that antibody-drug conjugates (ADCs) consisting of cAC10 (anti-CD30) linked to the antimitotic agent monomethylauristatin E (MMAE) lead to potent in vitro and in vivo activities against antigen positive tumor models. MMAF is a new antimitotic auristatin derivative with a charged C-terminal phenylalanine residue that attenuates its cytotoxic activity compared to its uncharged counterpart, MMAE, most likely due to impaired intracellular access. In vitro cytotoxicity studies indicated that mAb-maleimidocaproyl-valine-citrulline-p-aminobenzyloxycarbonyl-MMAF (mAb-L1-MMAF) conjugates were >2200-fold more potent than free MMAF on a large panel of CD30 positive hematologic cell lines. As with cAC10-L1-MMAE, the corresponding MMAF ADC induced cures and regressions of established xenograft tumors at well tolerated doses. To further optimize the ADC, several new linkers were generated in which various components within the L1 linker were either altered or deleted. One of the most promising linkers contained a noncleavable maleimidocaproyl (L4) spacer between the drug and the mAb. cAC10-L4-MMAF was approximately as potent in vitro as cAC10-L1-MMAF against a large panel of cell lines and was equally potent in vivo. Importantly, cAC10-L4-MMAF was tolerated at >3 times the MTD of cAC10-L1-MMAF. LCMS studies indicated that drug released from cAC10-L4-MMAF was the cysteine-L4-MMAF adduct, which likely arises from mAb degradation within the lysosomes of target cells. This new linker technology appears to be ideally suited for drugs that are both relatively cell-impermeable and tolerant of substitution with amino acids. Thus, alterations of the linker have pronounced impacts on toxicity and lead to new ADCs with greatly improved therapeutic indices.[4] |
| 分子式 |
C39H65N5O8
|
|---|---|
| 分子量 |
731.98
|
| 精确质量 |
731.483
|
| 元素分析 |
C, 64.00; H, 8.95; N, 9.57; O, 17.49
|
| CAS号 |
745017-94-1
|
| 相关CAS号 |
MMAF hydrochloride;1415246-68-2;MMAF-d8 hydrochloride;MMAF sodium;1799706-65-2;MMAF-OMe;863971-12-4;MMAF sodium;1799706-65-2;MMAF;745017-94-1;MMAF-d8
|
| PubChem CID |
10395173
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
896.8±65.0 °C at 760 mmHg
|
| 闪点 |
496.2±34.3 °C
|
| 蒸汽压 |
0.0±0.3 mmHg at 25°C
|
| 折射率 |
1.522
|
| LogP |
4.36
|
| tPSA |
173.59
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
21
|
| 重原子数目 |
52
|
| 分子复杂度/Complexity |
1160
|
| 定义原子立体中心数目 |
9
|
| SMILES |
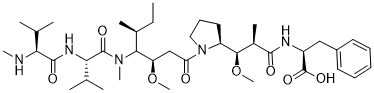
O(C([H])([H])[H])[C@]([H])([C@]([H])(C(N([H])[C@]([H])(C(=O)O[H])C([H])([H])C1C([H])=C([H])C([H])=C([H])C=1[H])=O)C([H])([H])[H])[C@]1([H])C([H])([H])C([H])([H])C([H])([H])N1C(C([H])([H])C([H])([C@]([H])([C@@]([H])(C([H])([H])[H])C([H])([H])C([H])([H])[H])N(C([H])([H])[H])C([C@]([H])(C([H])(C([H])([H])[H])C([H])([H])[H])N([H])C([C@]([H])(C([H])(C([H])([H])[H])C([H])([H])[H])N([H])C([H])([H])[H])=O)=O)OC([H])([H])[H])=O
|
| InChi Key |
MFRNYXJJRJQHNW-DEMKXPNLSA-N
|
| InChi Code |
InChI=1S/C39H65N5O8/c1-12-25(6)34(43(9)38(48)33(24(4)5)42-37(47)32(40-8)23(2)3)30(51-10)22-31(45)44-20-16-19-29(44)35(52-11)26(7)36(46)41-28(39(49)50)21-27-17-14-13-15-18-27/h13-15,17-18,23-26,28-30,32-35,40H,12,16,19-22H2,1-11H3,(H,41,46)(H,42,47)(H,49,50)/t25-,26+,28-,29-,30+,32-,33-,34-,35+/m0/s1
|
| 化学名 |
(2S)-2-[[(2R,3R)-3-methoxy-3-[(2S)-1-[(3R,4S,5S)-3-methoxy-5-methyl-4-[methyl-[(2S)-3-methyl-2-[[(2S)-3-methyl-2-(methylamino)butanoyl]amino]butanoyl]amino]heptanoyl]pyrrolidin-2-yl]-2-methylpropanoyl]amino]-3-phenylpropanoic acid
|
| 别名 |
MMAF; Monomethyl auristatin F; MMAF; 745017-94-1; Monomethyl Auristatin F; MMAF (GMP); (2S)-2-[[(2R,3R)-3-methoxy-3-[(2S)-1-[(3R,4S,5S)-3-methoxy-5-methyl-4-[methyl-[(2S)-3-methyl-2-[[(2S)-3-methyl-2-(methylamino)butanoyl]amino]butanoyl]amino]heptanoyl]pyrrolidin-2-yl]-2-methylpropanoyl]amino]-3-phenylpropanoic acid; MONOMETHYLAURISTATIN PHENYLALANINE; MFCD25976742; (S)-2-((2R,3R)-3-((S)-1-((3R,4S,5S)-4-((S)-N,3-Dimethyl-2-((S)-3-methyl-2-(methylamino)butanamido)butanamido)-3-methoxy-5-methylheptanoyl)pyrrolidin-2-yl)-3-methoxy-2-methylpropanamido)-3-phenylpropanoic acid; desmethyl-auristatin F;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: (1). 该产品在溶液状态不稳定,请现配现用。 (2). 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 100~140 mg/mL (136.6~191.3 mM)
Ethanol: ~100 mg/mL (~136.6 mM) Water: ~100 mg/mL (~136.6 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 3.5 mg/mL (4.78 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 35.0mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 3.5 mg/mL (4.78 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 35.0 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.3662 mL | 6.8308 mL | 13.6616 mL | |
| 5 mM | 0.2732 mL | 1.3662 mL | 2.7323 mL | |
| 10 mM | 0.1366 mL | 0.6831 mL | 1.3662 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|
|