| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
|
| 体内研究 (In Vivo) |
莫米松(0.1-3 mg/kg;鼻内)可降低气道对雾化醋甲胆碱的敏感性,并在最高测试剂量(3 mg/kg)下以剂量依赖性方式抑制轻度过敏性肺部炎症。过敏原诱导小鼠 Penh 升高 [3]。
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Nasal spray is virtually undetectable in plasma Metabolism / Metabolites Hepatic. Extensive metabolism to multiple metabolites. There are no major metabolites detectable in plasma. Upon in vitro incubation, one of the minor metabolites formed is 6ß-hydroxy-mometasone furoate. In human liver microsomes, the formation of the metabolite is regulated by cytochrome P-450 3A4. Biological Half-Life 5.8 hours |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Neither topical mometasone nor the mometasone nasal implant have been studied during breastfeeding. Since only extensive application of the most potent corticosteroids may cause systemic effects in the mother, it is unlikely that short-term application of topical corticosteroids or the slow-release implant would pose a risk to the breastfed infant by passage into breastmilk. However, it would be prudent to use the least potent drug on the smallest area of skin possible. It is particularly important to ensure that the infant's skin does not come into direct contact with the areas of skin that have been treated. Current guidelines allow topical corticosteroids to be applied to the nipples just after nursing for eczema, with the nipples cleaned gently before nursing. Only water-miscible cream or gel products should be applied to the breast because ointments may expose the infant to high levels of mineral paraffins via licking. ◉ Effects in Breastfed Infants Topical application of a corticosteroid with relatively high mineralocorticoid activity (isofluprednone acetate) to the mother's nipples resulted in prolonged QT interval, cushingoid appearance, severe hypertension, decreased growth and electrolyte abnormalities in her 2-month-old breastfed infant. The mother had used the cream since birth for painful nipples. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. ◉ Summary of Use during Lactation Neither inhaled mometasone nor mometasone nasal implants have been studied during breastfeeding. Although not measured, the amounts of inhaled and nasal corticosteroids absorbed into the maternal bloodstream and excreted into breastmilk are probably too small to affect a breastfed infant. Expert opinion considers inhaled, nasal and oral corticosteroids acceptable to use during breastfeeding. See also Mometasone, Topical. ◉ Effects in Breastfed Infants None reported with any corticosteroid. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding 98% to 99% (in a concentration range of 5 to 500 ng/mL). |
| 参考文献 |
|
| 其他信息 |
Mometasone is an 11beta-hydroxy steroid, a 17alpha-hydroxy steroid, a 20-oxo steroid, a 3-oxo-Delta(1),Delta(4)-steroid, a chlorinated steroid and a tertiary alpha-hydroxy ketone. It has a role as an anti-inflammatory drug, a dermatologic drug, a vasoconstrictor agent and an anti-allergic agent. It is functionally related to a Delta(1)-progesterone.
Mometasone is a corticosteroid not currently used in medical products. [Mometasone furoate] however, is still in use. Mometasone is a Corticosteroid. The mechanism of action of mometasone is as a Corticosteroid Hormone Receptor Agonist, and Corticosteroid Hormone Receptor Agonist. Mometasone is a synthetic topical glucocorticoid receptor (GR) agonist with anti-inflammatory, anti-pruritic and vasoconstrictive properties. Upon administration, mometasone binds to cytoplasmic GRs and subsequently activates GR-mediated gene expression. This results in the synthesis of certain anti-inflammatory proteins, while inhibiting the synthesis of certain inflammatory mediators. Specifically, mometasone appears to induce phospholipase A2 inhibitory proteins, thereby controlling the release of the inflammatory precursor arachidonic acid from phospholipid membrane by phospholipase A2. A pregnadienediol derivative ANTI-ALLERGIC AGENT and ANTI-INFLAMMATORY AGENT that is used in the management of ASTHMA and ALLERGIC RHINITIS. It is also used as a topical treatment for skin disorders. See also: Mometasone Furoate (has salt form); Mometasone Furoate Monohydrate (active moiety of). Drug Indication The inhaler is indicated for the maintenance treatment of asthma as prophylactic therapy. The nasal spray is indicated for the treatment of the nasal symptoms of seasonal allergic and perennial allergic rhinitis. Mechanism of Action Unbound corticosteroids cross cell membranes and bind with high affinity to specific cytoplasmic receptors. Inflammation is decreased by diminishing the release of leukocytic acid hydrolases, prevention of macrophage accumulation at inflamed sites, interference with leukocyte adhesion to the capillary wall, reduction of capillary membrane permeability, reduction of complement components, inhibition of histamine and kinin release, and interference with the formation of scar tissue. The antiinflammatory actions of corticosteroids are thought to involve phospholipase A2 inhibitory proteins, lipocortins, which control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes. Mometasone furoate has been shown in vitro to exhibit a binding affinity for the human glucocorticoid receptor which is approximately 12 times that of dexamethasone, 7 times that of triamcinolone acetonide, 5 times that of budesonide, and 1.5 times that of fluticasone. Pharmacodynamics Mometasone is a medium-potency synthetic corticosteroid with antiinflammatory, antipruritic, and vasoconstrictive properties. Studies in asthmatic patients have demonstrated that mometasone provides a favorable ratio of topical to systemic activity due to its primary local effect along with the extensive hepatic metabolism and the lack of active metabolites. Though effective for the treatment of asthma, glucocorticoids do not affect asthma symptoms immediately. Maximum improvement in symptoms following inhaled administration of mometasone furoate may not be achieved for 1 to 2 weeks or longer after starting treatment. When glucocorticoids are discontinued, asthma stability may persist for several days or longer. Mometasone has been shown in vitro to exhibit a binding affinity for the human glucocorticoid receptor which is approximately 12 times that of dexamethasone, 7 times that of triamcinolone acetonide, 5 times that of budesonide, and 1.5 times that of fluticasone. The clinical significance of these findings is unknown. |
| 分子式 |
C22H28O4CL2
|
|---|---|
| 分子量 |
427.36132
|
| 精确质量 |
520.142
|
| CAS号 |
105102-22-5
|
| 相关CAS号 |
Mometasone-d5
|
| PubChem CID |
441335
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.35g/cm3
|
| 沸点 |
586.6ºC at 760mmHg
|
| 熔点 |
220ºC (decomposes)
|
| 闪点 |
308.5ºC
|
| 蒸汽压 |
4.47E-18mmHg at 25°C
|
| 折射率 |
1.603
|
| LogP |
4.869
|
| tPSA |
93.81
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
28
|
| 分子复杂度/Complexity |
806
|
| 定义原子立体中心数目 |
8
|
| SMILES |
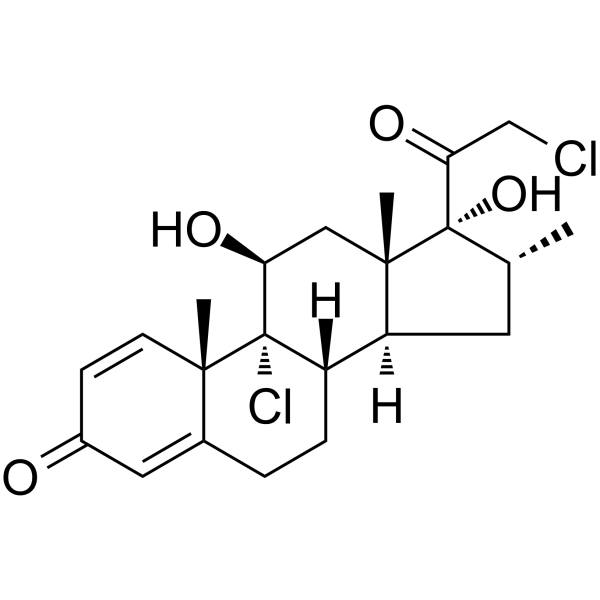
C[C@@H]1C[C@H]2[C@@H]3CCC4=CC(=O)C=C[C@@]4([C@]3([C@H](C[C@@]2([C@]1(C(=O)CCl)O)C)O)Cl)C
|
| InChi Key |
QLIIKPVHVRXHRI-CXSFZGCWSA-N
|
| InChi Code |
InChI=1S/C22H28Cl2O4/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,24)17(26)10-20(16,3)22(12,28)18(27)11-23/h6-7,9,12,15-17,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1
|
| 化学名 |
(8S,9R,10S,11S,13S,14S,16R,17R)-9-chloro-17-(2-chloroacetyl)-11,17-dihydroxy-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~125 mg/mL (~292.49 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.87 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.87 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (4.87 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3399 mL | 11.6997 mL | 23.3995 mL | |
| 5 mM | 0.4680 mL | 2.3399 mL | 4.6799 mL | |
| 10 mM | 0.2340 mL | 1.1700 mL | 2.3399 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Study to Compare the Pharmacokinetics of Fixed-Dose Combination of Mometasone + Azelastine Nasal Spray to Mometasone and Azelastine Nasal Sprays in Adolescents and Young Adults With Seasonal Allergic Rhinitis
CTID: NCT05887843
Phase: Phase 1 Status: Terminated
Date: 2023-09-28