| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
P2Y6 Receptor
The target of MRS 2578 is the purinergic receptor P2Y6, with an IC50 of 10.8 nM for human P2Y6 receptor and 9.1 nM for rat P2Y6 receptor [1] |
|---|---|
| 体外研究 (In Vitro) |
MRS2578 (1 μM) 完全阻止 1321N1 星形细胞瘤细胞在 TNFα 诱导的细胞凋亡过程中不受 UDP 保护[1]。在 HMEC-1 细胞中,MRS 2578 (10 μM) 完全消除 TNF-α 诱导的 NF-κB 报告基因活性。在 HMEC-1 细胞中,MRS 2578 (10 μM) 显着降低 TNF-α 诱导的促炎基因表达 [2]。
P2Y(6)核苷酸受体的生理作用可能涉及基于受体组织分布的心血管、免疫和消化功能,并且缺乏针对该受体的选择性拮抗剂。我们合成了一系列对称的芳基二异硫氰酸酯衍生物,并研究了它们抑制由五种重组P2Y受体亚型激活诱导的磷脂酶C(PLC)活性的能力。几种衍生物在抑制UDP对1321N1人星形胶质细胞中表达的人和大鼠P2Y(6)受体的作用方面比激活人P2Y(1)、P2Y(2)、P2X(4)和P2Y(11)受体更有效。1,2-二苯基乙烷(MRS2567)和1,4-二(苯基硫脲基)丁烷(MRS2578)的二异硫氰酸酯衍生物的抑制作用是浓度依赖性的,无法克服,IC(50)值分别为126+/-15 nM和37+/-16 nM(人)和101+/-27 nM和98+/-11 nM(大鼠)。1,4-苯二异硫氰酸酯衍生物(MRS2575)仅抑制人而不抑制大鼠P2Y(6)受体活性。MRS2567和MRS2578在10μM时不影响表达P2Y(2)和P2Y(4)受体的细胞的UTP(100nM)诱导的反应,也不影响P2Y(1)受体的2-甲硫基-ADP(30nM)诱导的响应或P2Y(11)受体的ATP(10μM)诱导反应。其他拮抗剂显示出混合选择性。选择性拮抗剂MRS2567、MRS2575和MRS2578(1microM)完全阻断了UDP对经历TNFα诱导凋亡的细胞的保护作用。因此,我们已经鉴定出P2Y(6)受体的强效、不可克服的拮抗剂,这些拮抗剂在PLC偶联的P2Y受体家族中具有选择性。[1] 选择性P2Y6受体拮抗剂MRS2578治疗以浓度依赖的方式抑制了机械牵张诱导的Rho活化,IC50值约为0.1μM(图5A和B)。[2] P2Y6受体拮抗剂MRS 2578在体外抑制介质诱导的血管内皮炎症[3] 在证明P2Y6受体转录物和蛋白质表达在炎症刺激下选择性增加后,我们接下来在体外研究了内皮P2Y6信号传导的功能后果。与体外模型一样,我们用含有p50/p65结合位点的NF-κB报告质粒转染HMEC-1细胞。用P2Y6激动剂尿苷二磷酸处理HMEC-1细胞仅导致NF-κB活性适度增加(1.64倍±0.45[倍;P<.05;补充图1)。然而,与之前关于P2Y6信号传导参与NF-κB活性的研究一致,26,27我们观察到在P2Y6拮抗剂MRS 2578存在的情况下,基础NF-κC活性受到严重抑制。NF-κB活性的降低与时间(图3A)和剂量(图3B)有关。因此,这些研究表明,P2Y6活化的下游靶点对于NF-κB活化是必要的,但还不够,并表明其在增强血管炎症方面具有间接作用[3]。 转染人或大鼠 P2Y6 受体的细胞实验中,MRS 2578 以浓度依赖性方式抑制 UDP 诱导的 P2Y6 受体激活,可阻断受体介导的细胞内 Ca²⁺ 浓度升高,且该抑制作用呈不可逆性 [1] - 选择性实验显示,MRS 2578 对其他嘌呤能受体(P2Y1、P2Y2、P2Y4、P2Y11、P2Y12、P2X1、P2X2、P2X4、P2X7)无明显抑制活性,即使浓度高达 10 μmol/L,对这些受体的抑制率仍低于 20% [1] - 人脐静脉内皮细胞(HUVECs)体外实验中,MRS 2578 预处理可显著抑制 UDP 诱导的炎症因子(IL-8、MCP-1、ICAM-1)mRNA 及蛋白表达,同时阻断 NF-κB 信号通路激活 [3] - 小鼠气道上皮细胞体外培养实验中,MRS 2578 可抑制 IL-4 诱导的 P2Y6 受体表达上调,减少趋化因子(CCL11、CCL24)的分泌,进而抑制嗜酸性粒细胞趋化 [4] - 大鼠心肌成纤维细胞实验中,MRS 2578 能阻断 P2Y6-Gα12/13 信号通路激活,抑制细胞增殖及胶原蛋白(Col1a1、Col3a1)的合成 [2] |
| 体内研究 (In Vivo) |
横主动脉缩窄 (TAC) 后,MRS2578(3 mg/kg;腹膜内注射;持续 3 天)可显着减少压力过载诱导的胶原沉积,而不影响心肌细胞肥大[4]。
抑制P2Y6受体可减轻体内压力超负荷诱导的心脏纤维化[2] 研究人员接下来研究了嘌呤能受体是否真的参与了体内压力超负荷诱导的心脏纤维化。TAC后使用MRS2578治疗显著抑制了压力超负荷诱导的胶原沉积,而不影响心肌细胞肥大(图6A-C)。MRS2578治疗显著抑制了压力超负荷引起的左心室功能障碍(图6D和E以及补充表3)。此外,MRS2578治疗抑制了压力超负荷引起的ANP、β-MHC、I型前胶原、骨膜炎蛋白和TGF-β2 mRNA表达的增加(图6F)。我们还发现,MRS2578抑制了压力超负荷诱导的Rho激活,TAC诱导骨膜炎蛋白、成熟TGF-βs和ACE蛋白表达的增加(图6G和H)。此外,我们发现苏拉明治疗还抑制了压力超负荷诱导的胶原沉积和左心室功能障碍(补充图7和补充表4)。这些结果表明,抑制P2Y6受体实际上可以减轻压力超负荷诱导的心脏纤维化和左心室功能障碍。 测量和主要结果:我们观察到气管内应用P2Y6R拮抗剂(MRS2578)和P2Y6R缺乏抑制了哮喘的主要特征,如支气管肺泡灌洗液嗜酸性粒细胞增多、气道重塑、Th2细胞因子产生和卵清蛋白-明矾模型中的支气管高反应性。在使用屋尘螨提取物诱导过敏性肺部炎症的模型中,MRS2578也能有效减少气道炎症。骨髓嵌合体实验揭示了P2Y6R在气道炎症中肺结构细胞表达的重要性。根据这一发现,我们发现实验性哮喘动物气道上皮细胞上P2Y6的表达强烈上调。关于潜在的机制,我们观察到MRS2578在体内抑制了肺上皮细胞释放IL-6和IL-8/KC,而肺内应用P2Y6R激动剂尿苷-5'-二磷酸会增加支气管肺泡中IL-6和KC的水平。此外,P2Y6受体的选择性激活在体外诱导小鼠和人肺上皮细胞释放IL-6和KC/IL-8。 结论:在急性和慢性过敏性气道炎症期间,气道上皮细胞上的P2Y6R表达上调,选择性阻断P2Y6R或P2Y6R缺乏对结构细胞的影响可减少实验性哮喘的主要特征。因此,阻断肺部P2Y6R可能是治疗过敏性气道炎症的靶点[4]。 小鼠压力超负荷心肌纤维化模型(主动脉缩窄术诱导)中,腹腔注射 MRS 2578(10 mg/kg,每日 1 次,持续 4 周)可显著降低心肌组织中胶原蛋白沉积,抑制成纤维细胞活化标志物(α-SMA)的表达,同时改善心脏舒张功能 [2] - 小鼠颈动脉结扎诱导的血管炎症模型中,MRS 2578 腹腔注射(10 mg/kg,每日 1 次,持续 14 天)可减少血管壁炎症细胞浸润(巨噬细胞、中性粒细胞),降低炎症因子(TNF-α、IL-6)及黏附分子(VCAM-1)的表达,抑制血管内膜增生 [3] - 小鼠过敏性气道炎症模型(卵清蛋白致敏激发)中,MRS 2578 腹腔注射(5 mg/kg,致敏后每 2 天 1 次,持续 14 天)可显著减轻气道嗜酸性粒细胞浸润,降低支气管肺泡灌洗液中 IL-4、IL-5、IL-13 等 Th2 细胞因子水平,改善气道高反应性及气道重塑(减少气道平滑肌增厚、黏液分泌)[4] |
| 酶活实验 |
P2Y6 受体活性抑制实验:将转染人或大鼠 P2Y6 受体的细胞接种于 96 孔板,培养至融合后加载 Ca²⁺ 荧光探针,加入不同浓度的 MRS 2578 预处理 30 分钟,再加入 UDP 刺激,通过荧光酶标仪实时检测细胞内 Ca²⁺ 荧光强度变化,计算 IC50 值 [1]
- 受体选择性实验:采用转染不同嘌呤能受体(P2Y1、P2Y2 等)的细胞,按上述 Ca²⁺ 检测方法,在 10 μmol/L 浓度下检测 MRS 2578 对各受体介导的 Ca²⁺ 响应的影响,评估其选择性 [1] |
| 细胞实验 |
核因子κB活性评价[3]
我们使用报告分析来评估核因子κB(NF-κB)活性。为了测量NF-κB的转录活性,将内皮细胞以2.5×104个细胞/孔的密度铺在24孔板上,并让其粘附过夜。然后根据制造商的说明,使用GeneJuice转染试剂,用0.25μg NF-κB启动子报告子或对照pGL3载体转染单层4小时。将细胞暴露于P2Y6受体拮抗剂MRS2578或溶剂(二甲亚砜[DMSO])30分钟。随后,再加入10ng/mL TNF-α2小时。在孵育期结束时,在冰冷的磷酸盐缓冲盐水中洗涤细胞两次,并使用萤光素酶测定系统测量萤光素酶活性。为了使蛋白质浓度正常化,使用BCA蛋白质测定试剂盒测定蛋白质浓度。 MRS2578抑制炎性细胞因子mRNA HMEC-1细胞与10μM MRS2578预孵育30分钟。加入TNF-α(10ng/mL),在指定时间点后裂解细胞。使用补充表1中总结的引物集测定NF-κB诱导基因的mRNA水平。 原代正常人支气管上皮细胞的分离。[4] 正常人支气管上皮细胞取自移植肺的支气管或其支气管环。这项研究得到了弗莱堡当地伦理委员会的批准。支气管被纵向切开,用一次性手术刀进行机械解剖,随后在冰冷的汉克斯平衡盐溶液中清洗。将粘膜切成小块,在37°C的水浴中用Dispase II在80 ml PII溶液中消化90分钟,补充100μl DNase、青霉素和链霉素。使用100μm的细胞过滤器过滤粗溶液,在4°C下以1500 rpm离心5分钟,然后重新悬浮在补充了青霉素和链霉素的RPMI 1649培养基中15分钟,并放置在培养皿中15分钟。仔细收获非贴壁细胞并计数,然后使用Quantum 286作为上皮细胞培养基在6孔板中培养(1×10~6个细胞/孔)。细胞休息24小时。然后更换培养基,用指定浓度的UDP和MRS2578刺激细胞。24小时后,收集细胞培养上清液,通过ELISA进行细胞因子测量。 人和小鼠上皮细胞系A549、BEAS-2B和LA-4。[4] 人细胞系细胞(BEAS-2B)在RPMI 1640中培养,补充10%胎牛血清(FCS)、100 U/ml庆大霉素和1%谷氨酰胺。LA-4小鼠支气管上皮细胞在补充了15%FCS、100U/ml庆大霉素和1%谷氨酰胺的F12K营养混合物中培养。A549细胞在补充了5%FCS、100U/ml庆大霉素和1%谷氨酰胺的Eagle最低必需培养基中生长。对于每个实验,将1×106个细胞接种到24孔板中并静置24小时。然后更换培养基,用指定浓度的UDP和MRS2578刺激细胞。24小时后,收集细胞培养上清液,通过ELISA进行细胞因子测量。 内皮细胞炎症反应实验:HUVECs 接种后培养至 80% 融合,用不同浓度的 MRS 2578(1、10、100 nM)预处理 1 小时,再加入 UDP 刺激 6 小时(检测 mRNA)或 24 小时(检测蛋白);采用实时定量 PCR 检测 IL-8、MCP-1、ICAM-1 的 mRNA 表达,Western blot 检测 NF-κB p65 磷酸化水平 [3] - 心肌成纤维细胞增殖及胶原合成实验:分离大鼠心肌成纤维细胞,接种后用 MRS 2578(100 nM)预处理 30 分钟,加入 UDP 刺激 48 小时;通过 CCK-8 法检测细胞增殖活性,实时定量 PCR 检测 Col1a1、Col3a1 的 mRNA 表达,免疫荧光检测 α-SMA 蛋白表达 [2] - 气道上皮细胞趋化因子分泌实验:小鼠气道上皮细胞接种后,用 MRS 2578(10、100 nM)预处理 1 小时,加入 IL-4 刺激 24 小时;采用 ELISA 法检测细胞培养上清中 CCL11、CCL24 的浓度,实时定量 PCR 检测 P2Y6 受体 mRNA 表达 [4] |
| 动物实验 |
Animal/Disease Models: 6weeks old male C57BL/6J mice[4]
Doses: 3 mg/kg Route of Administration: intraperitoneal (ip)injection; daily for 3 days after TAC Experimental Results: Dramatically suppressed pressure overload-induced collagen deposition. Animals and TAC surgery [2] Transgenic C57BL/6J mice expressing p115-RGS were tried to generate three times. We obtained only one line that was used in this study. Two lines of transgenic mice expressing CA-Gα13 were generated (lines 1 and 5). Heterozygote of line 5 was used in this study. Age-matched male WT C57BL/6J mice were used as control. TAC surgery was performed on 8- to 10-week-old male p115-Tg and WT C57BL/6J mice. A mini-osmotic pump (Alzet) filled with saline, MRS2578, or suramin was implanted intraperitoneally 3 days after TAC into 6-week-old male C57BL/6J mice. Details can be found in Supplementary methods at The EMBO Journal Online (http://embojournal.org). Murine endotoxinemia model [3] C57BL/6 mice or previously described P2Y6−/− mice on C57BL/6 background19 or corresponding littermate controls matched in age, sex, and weight were used. Mice were anesthetized, and 300 μg of LPS (Escherichia coli O26:B6) or vehicle were injected into the jugular vein. Where indicated, 100 μL of 10μM antagonist MRS2578 were given before and 1 hour after the application of LPS. All animal studies were approved by the local animal ethics committee and performed according to the respective guidelines. Ovalbumin/alum model of acute and chronic allergic airway inflammation. [4] Acute model: Female C57BL/6 mice, P2Y6R−/− and P2Y6R+/+ littermates on a C57Bl/6 background (n = 5 per group) were sham or ovalbumin (OVA) sensitized and challenged with OVA grade III, as previously published. The generation of P2Y6R−/− animals was previously described (12); animals were backcrossed to C57bl/6 background for at least eight generations. Briefly, mice were OVA or sham sensitized by intraperitoneal injection of OVA/alum or phosphate-buffered saline (PBS)/alum on Days 0 and 7 and were challenged with OVA aerosols on Days 17 to 19. Thirty minutes before each allergen challenge, animals were anesthetized with ketamine and xylazine and given an intratracheal injection of control vehicle or the receptor antagonist MRS2578 or the agonist UDP . Experiments were repeated three times. [4] Chronic model: Female C57BL/6 mice (6–9 wk, n = 8 per group) were sham or OVA sensitized by intraperitoneal injection on Day 0 and 7 and subsequently challenged with OVA aerosols three times weekly for 8 weeks. Treatment with MRS2578 was performed three times weekly for the last 2 weeks of OVA aerosol challenge, 30 minutes before each allergen challenge. Experiments were repeated three times. [4] Twenty-four hours after the last OVA exposure, in both acute and chronic OVA models, measurement of airway hyperresponsiveness, fluorescence-activated cell sorter analysis of the bronchoalveolar lavage fluid (BALF), and lung resection for histology and immunohistochemistry were performed as previously described. The levels of cytokines were measured in BALF and in restimulated mediastinal lymph nodes (MLN). For details about the airway hyperresponsiveness method, cytokine measurement, and histology and immunohistochemistry, see online supplement. House dust mite–induced allergic airway inflammation. [4] Female C57BL/6 mice (6–9 wk, n = 5 per group) were injected intratracheally with 100 μg Dermatophagoides pteronyssinus extract dissolved in 80 μl PBS on Day 0, Day 7, and Day 14. In the MRS2578-treated group, house dust mite extract was admixed with MRS2578 on Days 7 and 14. Animals were assessed for the classic features of asthma, such as airway hyperresponsiveness, inflammation, and remodeling, and cytokine levels in restimulated cells of MLN on day 17, as previously described. Experiments were repeated three times. For details, see online supplement. Mouse cardiac fibrosis experiment: 8-week-old C57BL/6 mice were subjected to transverse aortic constriction to establish a pressure overload model, while the sham-operated group only underwent thoracotomy without aortic constriction. From the first day after surgery, the model group was intraperitoneally injected with MRS 2578 (10 mg/kg), and the control group was injected with an equal volume of normal saline, once daily for 4 weeks. At the end of the experiment, cardiac function, myocardial collagen content, and related protein expression were detected [2] - Mouse vascular inflammation experiment: 8-week-old C57BL/6 mice were subjected to carotid artery ligation. From the first day after surgery, MRS 2578 (10 mg/kg) or normal saline was intraperitoneally injected once daily for 14 days. After the mice were sacrificed, the ligated carotid artery was isolated for tissue section staining to detect inflammatory cell infiltration and intimal hyperplasia, and real-time quantitative PCR was used to detect the expression of inflammatory factors in vascular tissue [3] - Mouse allergic airway inflammation experiment: 6-8-week-old BALB/c mice were sensitized by intraperitoneal injection of ovalbumin + aluminum adjuvant, and challenged by ovalbumin nebulization 14 days later to establish the model. From the first day after sensitization, the administration group was intraperitoneally injected with MRS 2578 (5 mg/kg) once every 2 days for 14 days. After the challenge, airway hyperresponsiveness, cell classification and cytokine levels in bronchoalveolar lavage fluid were detected, and the pathological changes of airway tissue were observed [4] |
| 毒性/毒理 (Toxicokinetics/TK) |
In in vitro experiments, MRS 2578 had no obvious cytotoxicity on P2Y6 receptor-transfected cells, HUVECs, cardiac fibroblasts, etc. at a concentration of 10 μmol/L, and the cell survival rate was higher than 90% [1][2][3]
- In in vivo experiments, after intraperitoneal injection of MRS 2578 in mice (maximum dose 10 mg/kg for 4 weeks), no significant weight loss, behavioral abnormalities, or increases in liver and kidney function indicators (ALT, AST, BUN, Cr) were observed [2][3][4] |
| 参考文献 |
|
| 其他信息 |
The present study demonstrated that MRS2567 and MRS2578 block agonist effects at both human and rat P2Y6 receptors, and MRS2575 selectively blocks effects at human P2Y6 but not rat P2Y6 receptors. This is the first report of selective antagonists for P2Y6 receptors, albeit insurmountable. Previous studies have shown that DIDS and H2DIDS block P2Y6 receptors at concentrations of 10–100 μM and are clearly less potent than MRS2567, MRS2578 and MRS2575. Compounds MRS2567 and MRS2578 can block UDP-stimulated activity at concentrations less than 1 μM (for human P2Y6 at IC50 values 126 ± 15 nM and 37 ± 16 nM, for rat P2Y6 at IC50 values 101 ± 27 nM and 98 ± 11 nM, respectively). Interestingly, MRS2575 is a selective antagonist for human P2Y6 receptors with an IC50 value of 155 ± 49 nM, while it has no effect on rat P2Y6 receptors. Since other compounds in this series inhibited other P2Y receptor subtypes, a panel of such diisothiocyanates may be useful in characterizing a given pharmacological response to extracellular nucleotides. For example, the following mixed selectivities were observed: MRS 2564 (P2Y6, P2Y11), MRS 2576 (P2Y1, P2Y2, P2Y4, P2Y6), and MRS 2577 (P2Y4, P2Y6).[1]
Recently it has been reported that P2Y2R and P2X7R signaling on hematopoietic cells (such as eosinophils and DCs) contributes to the development of allergic airway inflammation. Functional expression of P2Y6R has also been described on DCs, eosinophils, mast cells, monocytes, and neutrophils, suggesting that P2Y6R might also be involved in the pathogenesis of allergic airway inflammation by affecting the function of these hematopoietic cells. We therefore investigated the effect of an intratracheal application of the P2Y6R-specific antagonist MRS2578 in different mouse models of asthma. Indeed, MRS2578 reduced the several features of asthma in the acute and chronic OVA-alum model as well as in a model wherein allergic airway inflammation had been induced by a house dust mite extract. Similarly, P2Y6R deficiency confirmed the importance of P2Y6R in the modulation of allergic inflammation, because P2Y6R-deficient animals sensitized and challenged with OVA presented decreased numbers of eosinophils, lymphocytes, neutrophils, and macrophages in BALF, as well as a decreased production of IL-4, IL-5, and IL-13 by restimulated cells of mediastinal lymph nodes.[4] MRS 2578 is a potent and highly selective irreversible antagonist of the P2Y6 receptor. Its chemical structure is a diisothiocyanate derivative, which blocks UDP-mediated signal transduction by binding to specific sites of the P2Y6 receptor [1] - By inhibiting P2Y6 receptor-related inflammatory responses and fibroblast activation, MRS 2578 exerts a protective effect in disease models such as cardiac fibrosis, vascular inflammation, and allergic airway inflammation, and is an important tool drug for studying the physiological functions of the P2Y6 receptor and the pathogenesis of related diseases [2][3][4] - Its mechanism of action is closely related to blocking the P2Y6-Gα12/13-NF-κB signaling pathway, which can inhibit the release of downstream inflammatory factors, cell proliferation, and the synthesis of fibrosis-related proteins [2][3] |
| 分子式 |
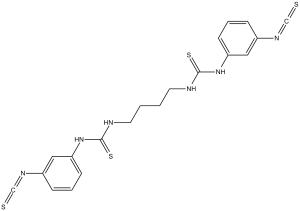
C20H20N6S4
|
|
|---|---|---|
| 分子量 |
472.67
|
|
| 精确质量 |
472.063
|
|
| 元素分析 |
C, 50.82; H, 4.27; N, 17.78; S, 27.13
|
|
| CAS号 |
711019-86-2
|
|
| 相关CAS号 |
|
|
| PubChem CID |
16078986
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
652.7±65.0 °C at 760 mmHg
|
|
| 闪点 |
348.5±34.3 °C
|
|
| 蒸汽压 |
0.0±2.0 mmHg at 25°C
|
|
| 折射率 |
1.687
|
|
| LogP |
5.1
|
|
| tPSA |
201.2
|
|
| 氢键供体(HBD)数目 |
4
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
9
|
|
| 重原子数目 |
30
|
|
| 分子复杂度/Complexity |
604
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
QOHNRGHTJPFMSL-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C20H20N6S4/c27-13-23-15-5-3-7-17(11-15)25-19(29)21-9-1-2-10-22-20(30)26-18-8-4-6-16(12-18)24-14-28/h3-8,11-12H,1-2,9-10H2,(H2,21,25,29)(H2,22,26,30)
|
|
| 化学名 |
1-(3-isothiocyanatophenyl)-3-[4-[(3-isothiocyanatophenyl)carbamothioylamino]butyl]thiourea
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.29 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (5.29 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (4.40 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 30% propylene glycol, 5% Tween 80, 65% D5W: 30 mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1156 mL | 10.5782 mL | 21.1564 mL | |
| 5 mM | 0.4231 mL | 2.1156 mL | 4.2313 mL | |
| 10 mM | 0.2116 mL | 1.0578 mL | 2.1156 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|