| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
| 体外研究 (In Vitro) |
莫匹罗星(BRL-4910A,假单酸)钙水合物(0-100 μM;48 小时)的 MIC 值范围为 0.06-0.25 μg/mL(MIC50 = 0.12 μg/mL,MIC90 = 0.25 μg/mL)对抗葡萄球菌、链球菌和一些革兰氏阴性菌[1]。人血清的存在会抑制麦吉罗星钙水合物的活性,因为其与人血清蛋白的 95% 强结合力[1]。
看来,麦吉罗星钙水合物的抗菌活性是通过可逆地抑制异亮氨酰转移 RNA 来实现的,进而抑制细菌蛋白质和RNA的合成[2]。 莫匹罗星钙水合物(2%软膏)降低肿瘤坏死因子-α(TNF-α)的表达,增加血管内皮生长因子的表达( VEGF),并降低促炎细胞因子 IL-1β 和 IL-17 的水平[4]。 麦吉罗星钙水合物的 MIC 分别为 0.25、1.26 和 1.59 mg/L,可抑制 MS(表皮葡萄球菌 ATCC 12228) )、MR (表皮葡萄球菌 (Se56-99)) 和 VIR (表皮葡萄球菌 (Se43-98))[5]。 |
|---|---|
| 体内研究 (In Vivo) |
MRSA:对甲氧西林耐药的金黄色葡萄球菌
口服和肠胃外给药后,莫匹罗星(BRL-4910A,假单胞酸)钙吸收良好;然而,抗生素长时间分解为无抗菌活性的代谢物莫酸 A 导致血清抗生素浓度短暂[1]。 使用任一局部治疗,麦罗星钙(2% 软膏;外用;每日两次;3) -6 d) 减少皮损中的总体细菌负荷[3]。 患有感染 MRSA 的压疮的小鼠每 4 天使用 2% 莫匹罗星钙软膏进行外部治疗[4]。 表皮葡萄球菌- 可以用麦吉罗星钙(100 mg/mL;皮下注射;7 d)预防血管假体移植物感染[5]。 |
| 细胞实验 |
细胞系:金黄色葡萄球菌
浓度:0-100 μM/mL 孵育时间:24、48 小时 结果:24 小时内减少 90–99%,MIC 和 MBC 值范围为 0.12 48 小时分别为 -1.0 μM/mL 和 4.0–32 μM/mL。 |
| 动物实验 |
Animal Model: MRSA skin infection model in mice (10-12 weeks old)[3]
Dosage: 2% ointment Administration: External administration; twice daily; 3-6 days Result: decreased the overall amount of bacteria present in the skin lesions, with reductions of 2.0 and 5.1 log10 CFU on days three and six, respectively. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Systemic or percutaneous absorption of mupirocin following dermal application is expected to be minimal in adults and children. Occlusive dressings do not significantly enhance drug absorption, but damaged skin may allow enhanced penetration of the drug across the skin barrier. Any mupirocin reaching the systemic circulation is rapidly metabolized to form the inactive monic acid, which is eliminated by renal excretion. Following the application of Centany (mupirocin ointment),2% to a 400 cm2 area on the back of 23 healthy volunteers once daily for 7 days, the mean (range) cumulative urinary excretion of monic acid over 24 hrs following the last administration was 1.25% (0.2% to 3.0%) of the administered dose of mupirocin. No information available. No information available. Metabolism / Metabolites Following intravenous or oral administration, mupirocin undergoes rapid hepatic metabolism to form the principal metabolite monic acid, which has no antibacterial activity. Biological Half-Life In healthy male volunteers, the elimination half-life of mupirocin was about 20 to 40 minutes following intravenous administration. The elimination half-life of monic acid was about 30 to 80 minutes. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Because less than 1% is absorbed after topical application, mupirocin is considered a low risk to the nursing infant.[1] Ensure that the infant's skin does not come into direct contact with the areas of skin that have been treated. Only water-miscible cream or gel products should be applied to the breast because ointments may expose the infant to high levels of mineral paraffins via licking.[2] Mupirocin applied topically to the nipples appears to be relatively ineffective as a treatment for sore, cracked nipples. ◉ Effects in Breastfed Infants A mother who was exclusively nursing her 52-day-old infant developed a soft-tissue infection. She was treated with intravenous teicoplanin 400 mg every 12 hours for 3 doses, then 400 mg daily for 5 days total, intravenous ceftriaxone 1 gram daily, topical mupirocin cream twice daily. A careful follow-up indicated that her infant had no adverse effects.[3] ◉ Effects on Lactation and Breastmilk A small, randomized, unblinded trial of mothers with sore, cracked nipples was performed. Mupirocin 2% applied to the nipples after each feeding was much less effective (16% vs 79%) than an oral antibiotic (cloxacillin or erythromycin for 10 days) in resolving the problem. Additionally, more patients' condition worsened (28% vs 5%) with mupirocin than with an oral antibiotic.[4] In a randomized, double-bind trial, lanolin was compared to an all-purpose nipple ointment containing mupirocin 1%, betamethasone 0.05%, and miconazole 2% for painful nipples while nursing in the first 2 weeks postpartum. The two treatments were equally effective in reducing nipple pain, nipple healing time, breastfeeding duration, breastfeeding exclusivity rate, mastitis and nipple symptoms, side effects or maternal satisfaction with treatment.[5] Protein Binding The protein binding of mupirocin is reported to be over 95%. |
| 参考文献 |
|
| 其他信息 |
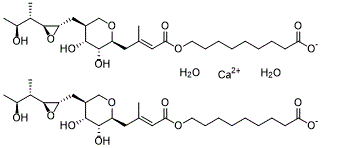
Mupirocin is an alpha,beta-unsaturated ester resulting from the formal condensation of the alcoholic hydroxy group of 9-hydroxynonanoic acid with the carboxy group of (2E)-4-[(2S)-tetrahydro-2H-pyran-2-yl]-3-methylbut-2-enoic acid in which the tetrahydropyranyl ring is substituted at positions 3 and 4 by hydroxy groups and at position 5 by a {(2S,3S)-3-[(2S,3S)-3-hydroxybutan-2-yl]oxiran-2-yl}methyl group. Originally isolated from the Gram-negative bacterium Pseudomonas fluorescens, it is used as a topical antibiotic for the treatment of Gram-positive bacterial infections. It has a role as a bacterial metabolite, an antibacterial drug and a protein synthesis inhibitor. It is a monocarboxylic acid, a member of oxanes, an epoxide, a secondary alcohol, a triol and an alpha,beta-unsaturated carboxylic ester. It is a conjugate acid of a mupirocin(1-).
Mupirocin, formerly termed pseudomonic acid A, is a novel antibacterial agent with a unique chemical structure and mode of action apart from other antibiotic agents. Produced by fermentation using the organism Pseudomonas fluorescens, mupirocin is a naturally-occurring antibiotic that displays a broad-specturm activity against many gram-positive bacteria and certain gram-negative bacteria in vitro. It primarily works by inhibiting bacterial protein synthesis. Due to its unique mode of action of inhibiting the activity of bacterial isoleucyl-tRNA synthetase, mupirocin does not demonstrate cross-resistance with other classes of antimicrobial agents, giving it a therapeutic advantage. It is available in topical formulations only due to extensive systemic metabolism and is used in the treatment of impetigo caused by Staphylococcus aureus and Streptococcus pyogenes and traumatic skin lesions due to secondary skin infections caused by S. aureus and S. pyogenes. There is also some clinical evidence that suggests the potential role of mupirocin in eradicating nasal carriage of Staphylococci when administered intranasally. Mupirocin is commonly marketed under the brand name Bactroban. Mupirocin is a RNA Synthetase Inhibitor Antibacterial. The mechanism of action of mupirocin is as a RNA Synthetase Inhibitor. Mupirocin has been reported in Pseudomonas fluorescens with data available. Mupirocin is a natural crotonic acid derivative extracted from Pseudomonas fluorescens. Mupirocin inhibits bacterial protein synthesis by specific reversible binding to bacterial isoleucyl tRNA synthase. With excellent activity against gram-positive staphylococci and streptococci, it is primarily used for treatment of primary and secondary skin disorders, nasal infections, and wound healing. (NCI04) A topically used antibiotic from a strain of Pseudomonas fluorescens. It has shown excellent activity against gram-positive staphylococci and streptococci. The antibiotic is used primarily for the treatment of primary and secondary skin disorders, nasal infections, and wound healing. Drug Indication Indicated for the treatment of impetigo and secondary skin infections, leading to traumatic skin lesions, due to _Staphylococcus aureus_ and _Streptococcus pyogenes_. Mechanism of Action Mupirocin specifically and reversibly binds to bacterial isoleucyl transfer-RNA (tRNA) synthetase, which is an enzyme that promotes the conversion of isoleucine and tRNA to isoleucyl-tRNA. Inhibition of this enzyme subsequently leads to the inhibition of the bacterial protein and RNA synthesis. Mupirocin is bacteriostatic at lower concentrations but it exerts bactericidal effects with prolonged exposure, killing 90-99% of susceptible bacteria over a 24 hour period. Pharmacodynamics Mupirocin is reported to be active against susceptible aerobic gram-positive cocci, such as _Staphylococcus aureus_, _Staphylococcus epidermidis_, and other beta-hemolytic streptococci_Streptococcus pyogenes_. It mediates its antibacterial activity by inhibiting the bacterial protein synthesis and formation of bacterial proteins essential for survival. The minimum bactericidal concentration (MBC) against relevant pathogens is generally eight-fold to thirty-fold higher than the minimum inhibitory concentration (MIC). In one clinical study investigating the therapeutic effectiveness of topical mupirocin in impetigo, the therapeutic response rate was about 94 to 98% after one week following the end of therapy. In clinical studies of patients with primary and secondary skin infections, both elimination of the bacterial pathogen and clinical cure or improvement hav been demonstrated in over 90% of patients receiving topical mupirocin. Mupirocin resistance as high as 81% has been reported previously. Resistance to mupirocin, which occurs more frequently in methicillin-resistant than methicillin-susceptible staphylococci, may occur with the production of a modified isoleucyl-tRNA synthetase, or the acquisition of, by genetic transfer, a plasmid mediating a new isoleucyl-tRNA synthetase. |
| 分子式 |
C52H90CAO20
|
|---|---|
| 分子量 |
1077.35288
|
| 精确质量 |
1074.57
|
| 元素分析 |
C, 58.08; H, 8.44; Ca, 3.73; O, 29.76
|
| CAS号 |
115074-43-6
|
| 相关CAS号 |
Mupirocin;12650-69-0;Mupirocin calcium;104486-81-9
|
| PubChem CID |
446596
|
| 外观&性状 |
White to off-white solid powder
|
| 沸点 |
672.3ºC at 760 mmHg
|
| 熔点 |
77-78
77 - 78 °C |
| 闪点 |
216.5ºC
|
| 蒸汽压 |
5.91E-21mmHg at 25°C
|
| LogP |
4.902
|
| tPSA |
288.56
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
17
|
| 重原子数目 |
35
|
| 分子复杂度/Complexity |
694
|
| 定义原子立体中心数目 |
8
|
| SMILES |
O.O.[Ca+2].OC(CCCCCCCCOC(/C=C(/CC1OCC(CC2OC2C(C(O)C)C)C(O)C1O)\C)=O)=O.OC(CCCCCCCCOC(/C=C(/CC1OCC(CC2OC2C(C(O)C)C)C(O)C1O)\C)=O)=O
|
| InChi Key |
DDHVILIIHBIMQU-YJGQQKNPSA-L
|
| InChi Code |
InChI=1S/2C26H44O9.Ca.2H2O/c2*1-16(13-23(30)33-11-9-7-5-4-6-8-10-22(28)29)12-20-25(32)24(31)19(15-34-20)14-21-26(35-21)17(2)18(3)27/h2*13,17-21,24-27,31-32H,4-12,14-15H2,1-3H3,(H,28,29)2*1H2/q+2/p-2/b2*16-13+/t2*17-,18-,19-,20-,21-,24+,25-,26-/m00.../s1
|
| 化学名 |
calcium 9-(((E)-4-((2S,3R,4R,5S)-3,4-dihydroxy-5-(((2S,3S)-3-((2S,3S)-3-hydroxybutan-2-yl)oxiran-2-yl)methyl)tetrahydro-2H-pyran-2-yl)-3-methylbut-2-enoyl)oxy)nonanoate dihydrate
|
| 别名 |
Mupirocin calcium salt; Calcium Mupirocin Dihydrate; Mupirocin Calcium Hydrate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~185.64 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1.25 mg/mL (2.32 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 12.5 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 1.25 mg/mL (2.32 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 12.5 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 1.25 mg/mL (2.32 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.9282 mL | 4.6410 mL | 9.2820 mL | |
| 5 mM | 0.1856 mL | 0.9282 mL | 1.8564 mL | |
| 10 mM | 0.0928 mL | 0.4641 mL | 0.9282 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Pharmacology of Mupirocin in Nasal Application in Healthy Volunteers: Monocentric Study
CTID: NCT06368856
Phase: Phase 1 Status: Recruiting
Date: 2024-04-16