| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|

| 靶点 |
FXR (EC50: 99 nM)
Farnesoid X Receptor (FXR):Obeticholic acid (6-ECDCA/INT-747) is a potent and selective FXR agonist with an EC50 of 99 nM in binding assays. It shows high specificity for FXR over other nuclear receptors. [1] |
||
|---|---|---|---|
| 体外研究 (In Vitro) |
- FXR激活与基因调控:Obeticholic acid(1 μM)在大鼠肝细胞中诱导小异源二聚体伴侣(SHP)和胆盐输出泵(BSEP)mRNA表达上调3-5倍,同时抑制胆固醇7α-羟化酶(CYP7A1)和Na+/牛磺胆酸盐协同转运肽(NTCP)表达70-80%。[2]
- 肠道屏障功能调节:在肠上皮细胞中,obeticholic acid(10 nM)增强claudin-1表达并减少claudin-2表达,使跨上皮电阻(TEER)较对照组提高40%。[4] 在大鼠肝细胞中,奥贝胆酸 (INT-747) 会提高 FXR 调节基因的表达[1]。奥贝胆酸 (INT-747) 可降低肝脏 JNK-1 和 JNK-2 表达[2]。在每个检查的菌株中,256 μg/mL 的奥贝胆酸 (INT-747) 完全抑制细菌生长。将INT-747添加到暴露于IFN-γ的Caco-2细胞的肠上皮中后,肠通透性没有改变[3]。 |
||
| 体内研究 (In Vivo) |
奥贝胆酸(INT-747)(10 毫克/公斤/天)完全逆转了 E217α 引起的胆汁淤积。通过提高 β-MCA、TCDCA 和 TDCA 的相对丰度,奥贝胆酸(INT-747)的给药可部分逆转 E217α 引起的总胆汁酸分泌受损[1]。在小鼠中,奥贝胆酸 (INT-747)7 (10 mg/kg) 和 HS 会加剧肺充血。在接受 HS 治疗的动物中,INT-747 不会改善肾脏病理学[2]。在 BDL 大鼠中,奥贝胆酸 (INT-747) (5 mg/kg) 显着提高了存活率。用奥贝胆酸 (INT-747) 治疗的 BDL 大鼠显示,仅在回肠中,闭孔claudin-1 的表达显着增加。使用 INT-747 治疗的 BDL 大鼠的回肠中 ZO-1 显着上调[3]。
细菌易位(BTL)驱动肝硬化的发病机制和并发症。法内脂x激活受体(FXR)是肝脏和肠道胆汁代谢的关键转录调节因子。我们在胆汁淤积性肝损伤大鼠模型中研究了潜在的肠道FXR功能障碍,并评估了FXR激动剂奥贝胆酸(INT-747)对肠道通透性、炎症和BTL的影响。大鼠在胆管结扎后10天内灌胃INT-747或载药,然后评估肠通透性、BTL、紧密连接蛋白表达、免疫细胞募集和回肠、肠系膜淋巴结和脾脏细胞因子表达的变化。辅助体外btl模拟实验采用Transwell支架。经载体处理的胆管结扎大鼠空肠和回肠FXR通路表达均下降,这与通过增加cladin -2表达增加肠道通透性有关,并与局部和全身自然杀伤细胞募集导致干扰素-γ表达增加和BTL增加有关。在INT-747治疗后,自然杀伤细胞和干扰素-γ的表达显著降低,与回肠选择性正常化通透性(上调claudin-1和occludin)和BTL显著降低有关。在体外,干扰素-γ诱导大肠杆菌易位增加,而不受INT-747的影响。在实验性胆汁淤积中,FXR激动剂通过减轻肠道炎症改善回肠屏障功能,导致BTL减少,从而证明了FXR在肠-肝轴中的重要保护作用。 - 雌激素诱导胆汁淤积模型保护作用:口服obeticholic acid(5 mg/kg/天,持续5天)可使雌激素诱导的胆汁淤积大鼠胆汁流量恢复至正常水平的85%,肝内胆汁酸蓄积减少60%,血清碱性磷酸酶(ALP)活性降低50%。[2] - 胆汁淤积大鼠肠道屏障保护:胆管结扎(BDL)大鼠中,obeticholic acid(5 mg/kg/每2天,持续10天)使肠系膜淋巴结细菌移位减少50%,回肠TEER提高30%,并与肠道干扰素-γ(IFN-γ)表达减弱相关。[4] - Dahl大鼠血压调节:高盐饮食Dahl大鼠每日口服obeticholic acid(10 mg/kg,持续4周)使收缩压降低18 mmHg,伴随肾脏二甲基精氨酸二甲胺水解酶(DDAH)表达上调2倍。[3] |
||
| 酶活实验 |
所有新化合物都通过建立的无细胞配体传感实验进行测试,该实验通过荧光共振能量转移来测量SRC1肽对FXR的配体依赖性募集结果如表1所示,奥比胆酸(INT-747;6-ECDCA (6b)是一种非常有效的FXR激动剂,EC50为99 nM。此外,6α-MeCDCA (6a)和6α-PrCDCA (6c)衍生物作为FXR激动剂表现出良好的效力,而6α-BnCDCA衍生物(6d)基本无活性。
在报告基因(hsp70EcRE)2-tk- luc,6a试验中,在HuH7细胞中使用全长人FXR, 6-ECDCA (6b)是一种有效的完全激动剂,EC50为85 nM(图2)。当在核受体LBD-GAL4嵌合受体的标准板上进行测试时,41 μM 6b仅激活FXR(LBD)-GAL4嵌合体(数据未显示)。其他受体在1 μM下未见明显活化。因此,6b是一种有效的选择性甾体FXR激动剂。[1]
- FXR激动活性检测: 1. 重组人FXR配体结合域与obeticholic acid(0.01–10 μM)及荧光配体置换探针共孵育。 2. 通过荧光偏振测量结合亲和力,EC50值由剂量-反应曲线计算。[1] |
||
| 细胞实验 |
肝细胞基因表达分析:
1. 原代大鼠肝细胞与obeticholic acid(0.1–10 μM)共孵育24小时。 2. 提取总RNA,通过qRT-PCR定量SHP、BSEP、CYP7A1和NTCP mRNA水平。[2] - 肠上皮屏障实验: 1. Caco-2细胞接种于Transwell小室,与obeticholic acid(1–100 nM)共孵育48小时。 2. 上皮电压计测量TEER,Western blot分析claudin-1/claudin-2蛋白表达。[4] 大鼠肝细胞暴露于1μM 6-ECDCA引起小异二聚体伴侣(Shp)和胆盐输出泵(bsep)mRNA的3-5倍诱导,胆固醇7α羟化酶(cyp7a1)、氧化甾醇12β羟化酶(cyp8b1)和Na(+)/牛磺胆酸盐共转运肽(ntcp)减少70-80%[2]。 |
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Obeticholic acid is absorbed in the gastrointestinal tract. The Cmax of obeticholic acid occurs at approximately 1.5 hours after an oral dose and ranges from 28.8-53.7 ng/mL at doses of 5-10mg. The median Tmax for both the conjugates of obeticholic acid is about 10 hours. One product monograph reports a Tmax of 4.5h for both 5 and 10mg doses. The AUC ranged from 236.6-568.1 ng/h/mL with 5mg to 10 mg doses. About 87% of an orally administered dose is accounted for in the feces. Less than 3% of the dose can be recovered in the urine. The volume of distribution of obeticholic acid is 618 L. Clearance information for obeticholic acid is not readily available in the literature. Metabolism / Metabolites The metabolism of obeticholic acid occurs in the liver. Obeticholic acid is conjugated with glycine or taurine, followed by secretion into bile. The conjugates are then absorbed in the small intestine and then re-enter the liver via enterohepatic circulation. The intestinal microbiota in the ileum converts conjugated obeticholic acid in a deconjugated form that may be either reabsorbed or eliminated. Glycine conjugates account for 13.8% of the metabolites and taurine conjugates account for 12.3%. Another metabolite, 3-glucuronide, may also be formed, but displays little pharmacological activity. Biological Half-Life The biological half-life of obeticholic acid is reported to be 24 hours. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Signs and Symptoms of Overdose For patients with primary biliary cholangitis, the administration of OCA at doses higher than the recommended maximum (ie, 25 mg or 50 mg once daily) results in dose-dependent hepatotoxicity, evidenced by elevations in ascites, portal hypertension, jaundice, and exacerbation of primary biliary cholangitis. Management of overdose There is no antidote for OCA. The FDA recommends closely monitoring the patient and providing appropriate care during an overdose. OCA should be administered only to patients who have an inadequate response or are intolerant to ursodeoxycholic acid alone. Hepatotoxicity In multiple preregistration clinical trials, obeticholic acid was found to decrease serum enzyme elevations in a high proportion of patients with different liver diseases. Instances of paradoxical worsening of liver disease or further increases in serum ALT or AST were not reported. However, the product label for obeticholic acid includes warnings that serious liver related adverse events occurred more commonly with active therapy than with placebo treatment. In a pooled analysis of 3 placebo controlled trials in patients with primary biliary cholangitis, liver related adverse events were 5.2 per 100 patient exposure years with 10 mg and 2.4 with placebo. Even higher rates occurred with higher doses of obeticholic acid: 19.8 per 100 patient years for 25 mg daily and 54.5 for 50 mg daily. The clinical features, timing of onset, pattern of enzyme elevations and course of these events were not described in detail. Within a little over a year after approval of obeticholic acid as therapy for primary biliary cholangitis, the FDA published a warning letter stating that they had received notification of 19 deaths and 11 cases of severe liver injury in patients taking obeticholic acid, most but not all of whom had preexisting cirrhosis (Case 1). More recently, severe instances of hepatic decompensation have been reported in patients with both primary biliary cholangitis as well as primary sclerosing cholangitis, two similar chronic cholestatic liver diseases. In patients with normal alkaline phosphatase levels, obeticholic therapy is associated with slight elevations in alkaline phosphatase, but without accompanying changes in serum aminotransferase levels, GGT or bilirubin, suggesting that the increases are due to alkaline phosphatase from other sources (bone, gastrointestinal tract). Therapy with OCA has been associated with development of pruritus in up to one-third of patients, but the appearance or worsening of itching is not usually associated with worsening of the underlying liver disease or increase in bilirubin or bile acid levels (other than OCA). Thus, obeticholic acid has apparent beneficial effects on liver test abnormalities, but has been linked to rare instances of worsening liver disease which may have clinical significance in patients with preexisting cirrhosis, particularly with use of higher doses of OCA. Adverse Effects The most common adverse effects associated with OCA administration include pruritus, fatigue, and abdominal pain and discomfort. Other reported adverse effects include rash, oropharyngeal pain, dizziness, constipation, arthralgia, dyslipidemia, headache, eczema, depression, hypersensitivity reactions, and abnormal thyroid function. The incidence of pruritus has been shown to increase in a dose-dependent manner and is increased when OCA is used as monotherapy. However, if a patient undergoes OCA therapy for 3 months without pruritus, this adverse effect is unlikely to occur subsequently. If pruritus does occur, it can be managed with bile acid sequestrants, antihistamines, dose reduction, or a temporary dosing interruption. Esophageal varices and ascites were also shown to occur during a 3-year interim analysis of patients in the POISE trial. Obeticholic acid is also associated with decreases in high-density lipoprotein cholesterol and triglycerides and increases in low-density lipoprotein (LDL) cholesterol. However, a double-blind, placebo-controlled study of patients with NASH showed that atorvastatin could be in combination with OCA to mitigate LDL changes. In patients with decompensated cirrhosis or Child-Pugh B or C hepatic impairment who receive more frequent dosing than the recommended starting dosage of 5 mg once weekly, hepatic decompensation and failure have been reported. Patients at risk for hepatic decompensation should be closely monitored while on OCA. Dose-dependent liver-related adverse reactions such as jaundice, worsening ascites, portal hypertension, and primary biliary cholangitis flare were also reported in patients with doses of 10 to 50 mg (5 times the recommended dose.) A pooled analysis of 3 placebo-controlled trials involving patients with PBC revealed that liver-related adverse effects occurred at a rate of 5.2 per 100 patient exposure years (PEY) for the 10 mg dose versus 2.4 for the placebo group. Liver-related adverse effects were 19.8 per 100 PEY for the 25 mg group and 54.5 per 100 PEY for the 50 mg group. Monitoring the patient's liver function during OCA therapy and liver-related adverse reactions is vital. Patients who experience paradoxical worsening of liver disease, progressive elevation of liver enzymes, or evidence of hepatic decompensation should discontinue OCA. Patients with cirrhosis presenting with portal hypertension should also discontinue OCA. Drug-Drug Interactions Bile acid binding resins (eg, cholestyramine, colestipol, colesevelam): Obeticholic acid absorption and effectiveness may be reduced if administered concurrently with bile acid binding resins. To minimize interaction, OCA should be taken at least 4 hours before or after these resins. Warfarin: Coadministration of OCA with warfarin can lower the international normalized ratio (INR). Monitoring INR levels and adjusting warfarin dosage may be necessary to maintain therapeutic efficacy. Inhibitors of bile salt efflux pump: Concomitant administration of OCA with bile salt efflux pump inhibitors (eg, cyclosporine) may lead to bile salt accumulation in the liver, potentially causing clinical symptoms. These combinations should be avoided. However, if BSEP inhibitors are necessary, serum transaminase and bilirubin levels should be monitored. CYP1A2 substrate: Obeticholic acid can potentially increase the exposure of CYP1A2 substrates. Serum drug concentrations should be observed in CYP1A2 substrates with narrow therapeutic indexes, such as theophylline and tizanidine. Protein Binding Obeticholic acid and its metabolic conjugates are >99% plasma protein-bound. |
||
| 参考文献 |
|
||
| 其他信息 |
- Mechanism of action:Obeticholic acid activates FXR to suppress bile acid synthesis (via SHP-mediated inhibition of CYP7A1) and enhance bile acid export (via BSEP upregulation), thereby reducing hepatic bile acid overload. [1][2]
- Therapeutic potential:Investigated for treating cholestatic liver diseases (e.g., primary biliary cholangitis) and improving gut-liver axis dysfunction in cholestasis. [2][4] - Formulation:Administered orally as a suspension in DMSO/PEG 300/Tween-80/saline for preclinical studies. [2][4] Pharmacodynamics The activation of the FXR by obeticholic acid acts to reduce the synthesis of bile acids, inflammation, and the resulting hepatic fibrosis. This may increase the survival of patients with PBC, but to date, an association between obeticholic acid and survival in PBC has not been established. Obeticholic acid is a dihydroxy-5beta-cholanic acid that is chenodeoxycholic acid carrying an additional ethyl substituent at the 6alpha-position. A semi-synthetic bile acid which acts as a farnesoid X receptor agonist and is used for treatment of primary biliary cholangitis. It has a role as a farnesoid X receptor agonist and a hepatoprotective agent. It is a dihydroxy-5beta-cholanic acid, a 3alpha-hydroxy steroid and a 7alpha-hydroxy steroid. It is functionally related to a chenodeoxycholic acid. Primary biliary cirrhosis, or PBC, is a progressive and chronic condition that leads to hepatic injury often resulting in end-stage liver failure that requires liver transplantation. Obeticholic acid is a farnesoid-X receptor (FXR) agonist used to treat this condition, possibly allowing for increased survival. In 2016, it was granted approval to treat primary biliary cholangitis in combination with [ursodeoxycholic acid], which was previously the mainstay treatment for this condition. In May 2021, the FDA updated its prescribing information to contraindicate the use of obeticholic acid in patients with PBC and advanced cirrhosis (e.g. those with portal hypertension or hepatic decompensation) due to a risk of liver failure, in some cases requiring liver transplantation. Obeticholic acid is currently being considered for FDA approval to treat fibrosis caused by non-alcoholic liver steatohepatitis (NASH). The NDA from Intercept Pharmaceuticals was approved in November 2019 and obeticholic acid is expected to be granted full approval for this indication in 2020. Obeticholic acid is a Farnesoid X Receptor Agonist. The mechanism of action of obeticholic acid is as a Farnesoid X Receptor Agonist. Obeticholic acid (OCA) is a synthetically modified bile acid and potent agonist of the farnesoid X nuclear receptor (FXR) that is used to treat liver diseases including primary biliary cholangitis. Obeticholic acid has not been linked to elevations in serum enzyme levels during therapy, but has been linked to an increased rate of severe liver related adverse events such as ascites, jaundice and liver failure. Obeticholic Acid is an orally bioavailable semi-synthetic bile acid derivative and an agonist of the nuclear bile acid receptor farnesoid X receptor (FXR) that may be used to lower hepatic exposure to bile acids. Upon oral administration, obeticholic acid targets and binds to FXR expressed in the liver and intestine, activating FXR-mediated bile acid, inflammatory, fibrotic, and metabolic pathways. This suppresses the production of bile acid in the hepatocytes and increases bile acid transport out of the hepatocytes, thereby reducing hepatic exposure to bile acids. FXR plays an important role in bile acid homeostasis and is involved in hepatic and intestinal inflammation and liver fibrosis. OBETICHOLIC ACID is a small molecule drug with a maximum clinical trial phase of IV (across all indications) that was first approved in 2016 and has 3 approved and 12 investigational indications. This drug has a black box warning from the FDA. |
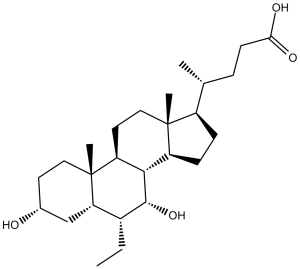
| 分子式 |
C26H44O4
|
|
|---|---|---|
| 分子量 |
420.63
|
|
| 精确质量 |
420.323
|
|
| 元素分析 |
C, 74.24; H, 10.54; O, 15.21
|
|
| CAS号 |
459789-99-2
|
|
| 相关CAS号 |
Obeticholic acid-d5;1992000-80-2;Obeticholic Acid-d4
|
|
| PubChem CID |
447715
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.1±0.1 g/cm3
|
|
| 沸点 |
562.9±25.0 °C at 760 mmHg
|
|
| 熔点 |
108-110
|
|
| 闪点 |
308.3±19.7 °C
|
|
| 蒸汽压 |
0.0±3.5 mmHg at 25°C
|
|
| 折射率 |
1.530
|
|
| LogP |
5.68
|
|
| tPSA |
77.76
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
4
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
30
|
|
| 分子复杂度/Complexity |
649
|
|
| 定义原子立体中心数目 |
11
|
|
| SMILES |
C[C@@]([C@]1([H])[C@@H](CC)[C@H]2O)(CC[C@@H](O)C1)[C@]3([H])[C@]2([H])[C@@](CC[C@]4([H])[C@H](C)CCC(O)=O)([H])[C@]4(C)CC3
|
|
| InChi Key |
ZXERDUOLZKYMJM-ZWECCWDJSA-N
|
|
| InChi Code |
InChI=1S/C26H44O4/c1-5-17-21-14-16(27)10-12-26(21,4)20-11-13-25(3)18(15(2)6-9-22(28)29)7-8-19(25)23(20)24(17)30/h15-21,23-24,27,30H,5-14H2,1-4H3,(H,28,29)/t15-,16-,17-,18-,19+,20+,21+,23+,24-,25-,26-/m1/s1
|
|
| 化学名 |
(4R)-4-[(3R,5S,6R,7R,8S,9S,10S,13R,14S,17R)-6-ethyl-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 5 mg/mL (11.89 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 50.0mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 5 mg/mL (11.89 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 50.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: ≥ 4.76 mg/mL (11.32 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.5 mg/mL (5.94 mM) (饱和度未知) in 10% EtOH + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL 澄清 EtOH 储备液加入400 μL PEG300 中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL 生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 5 中的溶解度: ≥ 2.5 mg/mL (5.94 mM) (饱和度未知) in 10% EtOH + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100μL 25.0mg/mL澄清EtOH储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 6 中的溶解度: ≥ 2.5 mg/mL (5.94 mM) (饱和度未知) in 10% EtOH + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清乙醇储备液加入到 900 μL 玉米油中并混合均匀。 配方 7 中的溶解度: ≥ 2.5 mg/mL (5.94 mM) (饱和度未知) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 8 中的溶解度: 5 mg/mL (11.89 mM) in 1% Methylcellulose(MC) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3774 mL | 11.8869 mL | 23.7739 mL | |
| 5 mM | 0.4755 mL | 2.3774 mL | 4.7548 mL | |
| 10 mM | 0.2377 mL | 1.1887 mL | 2.3774 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|---|
|