| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
Tetracycline; protein synthesis of bacteria
With MIC90s of 1.0, 0.25, 0.5, 0.25, and 2.0 μg/mL, respectively, omadacycline shows activity against methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), beta-hemolytic streptococci, penicillin-resistant Streptococcus pneumonia (PRSP), and Haemophilus influenzae (H. influenzae)[2]. Through ribosomal protection and active tetracycline efflux, omadacycline effectively combats strains that express resistance to tetracycline and other antibiotics[2]. |
|---|---|
| 体外研究 (In Vitro) |
体外活性:Omadacycline 是一种新型氨甲基四环素抗生素,正在开发用于口服和静脉 (IV) 给药,用于治疗社区获得性细菌感染,例如急性细菌性皮肤和皮肤结构感染 (ABSSSI)、社区获得性细菌性肺炎 (CABP)和尿路感染(UTI)。在体外,omadacycline 对革兰氏阳性和革兰氏阴性需氧菌、厌氧菌和非典型病原体(包括军团菌和衣原体属)具有活性。奥马达环素提供每日一次的口服和静脉注射给药方式,其临床耐受性和安全性与当前用于治疗严重社区获得性感染的抗生素相比具有优势,而在这些感染中,耐药性已大大降低了有效性。在针对复杂皮肤和皮肤结构感染(包括 MRSA 感染患者)的研究中,omadacycline 表现出与利奈唑胺相当的疗效和耐受性。正在进行和计划中的临床研究正在评估 omadacycline 作为治疗严重社区获得性细菌感染的单一疗法,包括急性细菌性皮肤和皮肤结构感染 (ABSSSI) 和社区获得性细菌性肺炎 (CABP)。本综述概述了奥马环素的发现、微生物学、非临床数据以及可用的临床安全性和有效性数据,并参考了其他当代四环素衍生抗生素。细胞测定:omadacycline 对 MRSA、VRE 和 β-溶血性链球菌的 MIC90 分别为 1.0 μg/mL、0.25 μg/mL 和 0.5 μg/mL,omadacycline 对 PRSP 和流感嗜血杆菌的 MIC90 为 0.25 μg/ml分别为2.0μg/mL和2.0μg/mL。 Omadacycline 对生物体具有活性,表现出两种主要的耐药机制:核糖体保护和活性四环素外排[1]。 Omadacycline 抑制蛋白质合成,但对 RNA、DNA 和肽聚糖合成无明显影响。此外,omadacycline 与细菌核糖体 30S 亚基上的四环素结合位点结合,基于额外的分子相互作用,其结合增强,类似于替加环素。
Omadacycline在体外对多种细菌显示出强大的抗菌活性。针对革兰氏阳性菌,它对金黄色葡萄球菌(包括耐甲氧西林MRSA)具有活性,对携带tet(M)基因的菌株MIC范围为0.125–1 µg/mL,对携带tet(K)基因的菌株为0.125–0.25 µg/mL。对于粪肠球菌和尿肠球菌(包括耐万古霉素菌株),针对核糖体保护菌株的MIC低至0.125–0.5 µg/mL,针对外排菌株为0.25 µg/mL。针对肺炎链球菌(包括耐青霉素和耐大环内酯类菌株),MIC值≤0.06 µg/mL。对于革兰氏阴性菌,如携带tet(A)外排基因的大肠杆菌,MIC为2 µg/mL。它还显示出对厌氧菌(例如艰难梭菌MIC范围0.25-8 µg/mL)、非典型病原体(例如嗜肺军团菌MIC范围0.06–1 µg/mL;肺炎衣原体MIC范围0.03–0.5 µg/mL)和快速生长分枝杆菌(例如脓肿分枝杆菌MIC50为1 µg/mL,MIC90为2 µg/mL)的活性。其活性基本不受常见四环素耐药决定簇的影响。[1] |
| 体内研究 (In Vivo) |
使用小鼠腹膜内感染模型证明了omadacycline的体内功效。单次静脉注射剂量的 omadacycline 对肺炎链球菌、大肠杆菌和金黄色葡萄球菌(包括含有 tet (M) 和 tet (K) 外排的菌株和 MRSA 菌株)具有疗效。获得的肺炎链球菌的50%有效剂量(ED50)范围为0.45 mg/kg至3.39 mg/kg,获得的金黄色葡萄球菌的ED50范围为0.30 mg/kg至1.74 mg/kg,大肠杆菌的ED50为2.02毫克/公斤。
使用小鼠腹腔感染模型证明了奥美他环素的体内疗效。单次静脉注射奥马达环素对肺炎链球菌、大肠杆菌和金黄色葡萄球菌具有疗效,包括含有tet(M)和tet(K)外排的菌株和MRSA菌株。获得的肺炎链球菌的50%有效剂量(ED50s)范围为0.45 mg/kg至3.39 mg/kg,获得的金黄色葡萄球菌的ED50s范围为0.30 mg/kg至1.74 mg/kg,大肠杆菌的ED50为2.02 mg/kg。这些结果表明了强大的体内疗效,包括对含有常见耐药决定因素的菌株的活性。奥马达环素在体外表现出对多种革兰氏阳性和选择性革兰氏阴性病原体的活性,包括含有耐药性决定簇的菌株,这种活性在体内转化为强效疗效[2]。 临床研究(II期和III期)证明了omadacycline在患者体内的有效性。在针对急性细菌性皮肤和皮肤结构感染的OASIS-1和OASIS-2试验中,静脉注射(IV)或口服奥玛环素不劣于利奈唑胺,对包括MRSA在内的病原体具有较高的临床成功率。在针对社区获得性细菌性肺炎的OPTIC试验中,IV奥玛环素不劣于莫西沙星。[1] |
| 酶活实验 |
奥马环素的体外稳定性及药物相互作用潜力[2]
测定了4.8 μM和48 μM的奥马环素在人微粒体和肝细胞中的稳定性。奥马大环素在人微粒体中孵育30分钟后,>90%的奥马环素被完整地回收。同样,奥马环素在人肝细胞中孵育24小时后,>86%的细胞恢复完好。这些结果表明,奥马环素没有代谢到任何显著程度。使用混合人肝微粒体制剂、S9、肝细胞质或重组黄素单加氧酶(FMO1、FMO3、FMO5)评估与奥马环素药物相互作用的可能性。在原代人肝细胞中,用1-100 μM的奥马环素和底物探针孵育24和48小时,评估CYP450同工酶的诱导作用。在浓度为1-50 μM的奥马环素和浓度近似于每个底物Km的同工酶特异性底物的混合人微粒体中,评估CYP450同工酶的抑制作用。评估的同工酶包括CYP 1A1、1A2、1B1、2A6、2B6、2C8、2C9、2C19、2D6、2E1、2J2和3A4/5。奥马环素没有诱导CYP同工酶,并且没有或很少(<40%的最大阳性对照反应)诱导它们的mrna。奥马达环素对CYP同工酶活性无明显抑制作用。此外,奥马环素及其可能的代谢物对CYP1A2 2C9、2D6或3A4/5没有时间依赖性的抑制作用。 Bioorg Med Chem.2016 Dec 15;24(24):6409-6419.
|
| 细胞实验 |
omadacycline 对 MRSA、VRE 和 β-溶血性链球菌的 MIC90 分别为 1.0 μg/mL、0.25 μg/mL 和 0.5 μg/mL,omadacycline 对 PRSP 和流感嗜血杆菌的 MIC90 分别为 0.25 μg/ml 和 2.0 μg /mL,分别。 Omadacycline 对生物体具有活性,表现出两种主要的耐药机制:核糖体保护和活性四环素外流。 Omadacycline 抑制蛋白质合成,但对 RNA、DNA 和肽聚糖合成无明显影响。此外,omadacycline 与细菌核糖体 30S 亚基上的四环素结合位点结合,基于额外的分子相互作用,其结合增强,类似于替加环素。
|
| 动物实验 |
Mice: Sterile saline is used to dissolve omadacycline. A 3-mL lock-top sterile syringe with a sterile 25-gauge, 5/8-in. needle is used to infect mice. Mice are given an intravenous (i.v.) dose of omadacycline or relevant comparator compounds at a volume of 10 ml/kg at one hour post-infection (p.i.). Each experiment involves testing a minimum of four dose levels on five mice per group. With a few notable exceptions, the usual dose range tested is 0.11 to 18 mg/kg of body weight[1]. Significantly higher or lower doses are needed for comparators to achieve 50% efficacy.
Systemic i.p. challenge model. Six-week-old, specific-pathogen-free, male CD-1 mice, weighing 18 to 30 g were used for all experiments. At 1 h postinfection (p.i.), mice were dosed intravenously (i.v.) with omadacycline or comparator compounds of interest, dissolved in sterile saline for injection at a volume of 10 ml/kg. All drug doses were formulated fresh immediately prior to administration and adjusted to account for percent activity. A minimum of four dose levels were tested per experiment with 5 mice/group. The typical doses tested ranged from 0.11 to 18 mg/kg of body weight, with exceptions for comparators that required significantly higher or lower doses to achieve 50% efficacy (dose range minimum-maximum, 0.08 to 54 mg/kg). Each study also included an untreated control group. Mice were housed in filter-topped cages in an isolated room and monitored for morbidity at least every 24 h for 7 days. Efficacy was determined by calculating the 50% effective dose (ED50) for all drugs tested. The ED50 is defined as the dose required to achieve 50% survival at 7 days p.i. and was estimated when possible using the formula y = 1/[1 + 10(log(k)-log(x)× 4.2)], where k = 0.5, by nonlinear regression analysis with Prism, version 3.0 software. [2] The provided protocols are from human clinical trials. For example, in the OASIS-1 skin infection study, patients received an IV loading dose of 100 mg omadacycline twice on day one, followed by 100 mg IV once daily, with an option to switch to oral 300 mg once daily. The comparator was linezolid 600 mg IV/oral twice daily. Treatment duration was 7-14 days. [1] In the OPTIC pneumonia study, patients received IV omadacycline 100 mg twice on day one, then 100 mg IV once daily, with an option to switch to oral therapy. The comparator was moxifloxacin 400 mg IV/oral once daily. [1] |
| 药代性质 (ADME/PK) |
The pharmacokinetics of omadacycline are best described by a linear, three-compartment model following a zero-order intravenous infusion or first-order oral administration with transit compartments to account for delayed absorption. Omadacycline has a volume of distribution (Vd) ranging from 190 to 204 L, a terminal elimination half-life (t½) of 13.5-17.1 h, total clearance (CLT) of 8.8-10.6 L/h, and protein binding of 21.3% in healthy subjects. Oral bioavailability of omadacycline is estimated to be 34.5%. A single oral dose of 300 mg (bioequivalent to 100 mg IV) of omadacycline administered to fasted subjects achieved a maximum plasma concentration (Cmax) of 0.5-0.6 mg/L and an area under the plasma concentration-time curve from 0 to infinity (AUC0-∞) of 9.6-11.9 mg h/L. The free plasma area under concentration-time curve divided by the minimum inhibitory concentration (i.e., fAUC24h/MIC), has been established as the pharmacodynamic parameter predictive of omadacycline antibacterial efficacy. Several animal models including neutropenic murine lung infection, thigh infection, and intraperitoneal challenge model have documented the in vivo antibacterial efficacy of omadacycline. A phase II clinical trial on complicated skin and skin structure infection (cSSSI) and three phase III clinical trials on ABSSSI and CABP demonstrated the safety and efficacy of omadacycline. The phase III trials, OASIS-1 (ABSSSI), OASIS-2 (ABSSSI), and OPTIC (CABP), established non-inferiority of omadacycline to linezolid (OASIS-1, OASIS-2) and moxifloxacin (OPTIC), respectively. Omadacycline is currently approved by the FDA for use in treatment of ABSSSI and CABP. Phase II clinical trials involving patients with acute cystitis and acute pyelonephritis are in progress. Mild, transient gastrointestinal events are the predominant adverse effects associated with use of omadacycline. Based on clinical trial data to date, the adverse effect profile of omadacycline is similar to studied comparators, linezolid and moxifloxacin. Unlike tigecycline and eravacycline, omadacycline has an oral formulation that allows for step-down therapy from the intravenous formulation, potentially facilitating earlier hospital discharge, outpatient therapy, and cost savings. Omadacycline has a potential role as part of an antimicrobial stewardship program in the treatment of patients with infections caused by antibiotic-resistant and multidrug-resistant Gram-positive [including methicillin-resistant Staphylococcus aureus (MRSA)] and Gram-negative pathogens. [https://pubmed.ncbi.nlm.nih.gov/31970713/]
Phase I studies indicated that omadacycline is administered once daily, either orally or intravenously. Doses higher than 300 mg IV led to a reversible increase in alanine aminotransferase. Oral doses higher than 400 mg led to mild nausea. Specific PK parameters such as half-life, bioavailability, absorption, distribution, metabolism, and excretion are not detailed in this reference. [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation No information is available on the use of omadacycline during breastfeeding. It is unknown how much omadacycline is excreted into breastmilk, but the drug is only about 35% absorbed orally under optimal circumstances, and is probably less from milk because of its calcium content. The manufacturer states that breastfeeding is not recommended during treatment and for 4 days after the last dose. If an infant is breastfed, monitor the infant for possible effects on the gastrointestinal flora, such as diarrhea, candidiasis (e.g., thrush, diaper rash) or rarely, blood in the stool indicating possible antibiotic-associated colitis. As a theoretical precaution, avoid prolonged or repeat courses during nursing. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. In clinical studies, omadacycline was generally well-tolerated. Common side effects included mild and transient nausea, especially with higher oral doses. Intravenous administration at doses >300 mg was associated with reversible increases in alanine aminotransferase. In a phase I study in women with cystitis, gastrointestinal side effects (vomiting, nausea) were higher than expected but were mild and did not lead to trial withdrawal. No information on LD50, organ toxicity, drug-drug interactions, or plasma protein binding is provided. [1] |
| 参考文献 | |
| 其他信息 |
Omadacycline (Nuzyra®) is a new aminomethylcycline, approved by the U. S. Food and Drug Administration in 2018, as a tetracycline antibacterial. It can be used in community-acquired pneumonia and in acute bacterial skin and skin-structure infections. It was developed and is commercialized by Paratek Pharmaceuticals. It is a semisynthetic compound, derived from minocycline, capable of evading widely distributed efflux and target protection antibacterial resistance mechanisms and has demonstrated activity in a broad spectrum of bacteria.[1]
\n\nOmadacycline is the first intravenous and oral 9-aminomethylcycline in clinical development for use against multiple infectious diseases including acute bacterial skin and skin structure infections (ABSSSI), community-acquired bacterial pneumonia (CABP), and urinary tract infections (UTI). The comparative in vitro activity of omadacycline was determined against a broad panel of Gram-positive clinical isolates, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), Lancefield groups A and B beta-hemolytic streptococci, penicillin-resistant Streptococcus pneumoniae (PRSP), and Haemophilus influenzae (H. influenzae). The omadacycline MIC90s for MRSA, VRE, and beta-hemolytic streptococci were 1.0 μg/ml, 0.25 μg/ml, and 0.5 μg/ml, respectively, and the omadacycline MIC90s for PRSP and H. influenzae were 0.25 μg/ml and 2.0 μg/ml, respectively. Omadacycline was active against organisms demonstrating the two major mechanisms of resistance, ribosomal protection and active tetracycline efflux. In vivo efficacy of omadacycline was demonstrated using an intraperitoneal infection model in mice. A single intravenous dose of omadacycline exhibited efficacy against Streptococcus pneumoniae, Escherichia coli, and Staphylococcus aureus, including tet(M) and tet(K) efflux-containing strains and MRSA strains. The 50% effective doses (ED50s) for Streptococcus pneumoniae obtained ranged from 0.45 mg/kg to 3.39 mg/kg, the ED50s for Staphylococcus aureus obtained ranged from 0.30 mg/kg to 1.74 mg/kg, and the ED50 for Escherichia coli was 2.02 mg/kg. These results demonstrate potent in vivo efficacy including activity against strains containing common resistance determinants. Omadacycline demonstrated in vitro activity against a broad range of Gram-positive and select Gram-negative pathogens, including resistance determinant-containing strains, and this activity translated to potent efficacy in vivo.[2] \n\nOmadacycline is a novel aminomethylcycline antibiotic developed as a once-daily, intravenous and oral treatment for acute bacterial skin and skin structure infection (ABSSSI) and community-acquired bacterial pneumonia (CABP). Omadacycline, a derivative of minocycline, has a chemical structure similar to tigecycline with an alkylaminomethyl group replacing the glycylamido group at the C-9 position of the D-ring of the tetracycline core. Similar to other tetracyclines, omadacycline inhibits bacterial protein synthesis by binding to the 30S ribosomal subunit. Omadacycline possesses broad-spectrum antibacterial activity against Gram-positive and Gram-negative aerobic, anaerobic, and atypical bacteria. Omadacycline remains active against bacterial isolates possessing common tetracycline resistance mechanisms such as efflux pumps (e.g., TetK) and ribosomal protection proteins (e.g., TetM) as well as in the presence of resistance mechanisms to other antibiotic classes.[3] \n\nParatek Pharmaceuticals are developing omadacycline (NUZYRA™), a first-in-class orally active aminomethylcycline antibacterial, as a treatment for various bacterial infections. The drug, which is available in intravenous and oral formulations, has a broad spectrum of antibacterial activity and was recently approved in the USA as a treatment for the treatment of community acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI) in adults. This article summarizes the milestones in the development of omadacycline leading to this first global approval for the treatment of CABP and ABSSSI.[4] Omadacycline (Nuzyra®) is a novel aminomethylcycline antibiotic, a semisynthetic derivative of minocycline, approved by the FDA in 2018. It is indicated for the treatment of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections. Its key advantage is the ability to circumvent major tetracycline resistance mechanisms (efflux and ribosomal protection), maintaining activity against many multidrug-resistant bacteria. It is administered once daily, offering a convenience advantage over some older tetracyclines. Clinical trials for its use in urinary tract infections (cystitis and acute pyelonephritis) are ongoing. [1] |
| 分子式 |
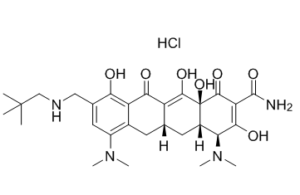
C29H41CLN4O7
|
|---|---|
| 分子量 |
593.1114
|
| 精确质量 |
592.266
|
| 元素分析 |
C, 58.73; H, 6.97; Cl, 5.98; N, 9.45; O, 18.88
|
| CAS号 |
1196800-39-1
|
| 相关CAS号 |
Omadacycline;389139-89-3;Omadacycline tosylate;1075240-43-5;Omadacycline-d9;2272886-41-4;Omadacycline mesylate;1196800-40-4
|
| PubChem CID |
54746487
|
| 外观&性状 |
Brown to black solid powder
|
| tPSA |
177Ų
|
| 氢键供体(HBD)数目 |
7
|
| 氢键受体(HBA)数目 |
10
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
41
|
| 分子复杂度/Complexity |
1140
|
| 定义原子立体中心数目 |
4
|
| SMILES |
CC(C)(C)CNCC1=CC(=C2C[C@H]3C[C@H]4[C@@H](C(=O)C(=C([C@]4(C(=O)C3=C(C2=C1O)O)O)O)C(=O)N)N(C)C)N(C)C.Cl
|
| InChi Key |
HXMCZSICOWSBRX-XGLFQKEBSA-N
|
| InChi Code |
InChI=1S/C29H40N4O7.ClH/c1-28(2,3)12-31-11-14-10-17(32(4)5)15-8-13-9-16-21(33(6)7)24(36)20(27(30)39)26(38)29(16,40)25(37)18(13)23(35)19(15)22(14)34;/h10,13,16,21,31,34,36-37,40H,8-9,11-12H2,1-7H3,(H2,30,39);1H/t13-,16-,21-,29-;/m0./s1
|
| 化学名 |
(4S,4aS,5aR,12aS)-4,7-bis(dimethylamino)-3,10,12,12a-tetrahydroxy-9-((neopentylamino)methyl)-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide hydrochloride
|
| 别名 |
Omadacycline HCl; Omadacycline hydrochloride; PTK 0796; PTK-0796; PTK0796; Amadacyclin; Omadacycline (hydrochloride); (4S,4aS,5aR,12aR)-4,7-bis(dimethylamino)-9-[(2,2-dimethylpropylamino)methyl]-1,10,11,12a-tetrahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4H-tetracene-2-carboxamide;hydrochloride; PTK0796 hydrochloride; CHEMBL3942449; Omadacycline; Nuzyra.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : 200 mg/mL (~!337.21 mM)
DMSO : 50 mg/mL (~84.30 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.62 mg/mL (4.42 mM) (饱和度未知) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
*20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: 50 mg/mL (84.30 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6860 mL | 8.4301 mL | 16.8603 mL | |
| 5 mM | 0.3372 mL | 1.6860 mL | 3.3721 mL | |
| 10 mM | 0.1686 mL | 0.8430 mL | 1.6860 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。