| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| Other Sizes |
|
| 靶点 |
D3 receptor
|
|---|---|
| 体外研究 (In Vitro) |
(+)-PD 128907 的选择性比人类 (Ki=179 nM) 和大鼠多巴胺 D2 受体高 100 倍,比 [3H]螺哌酮与多巴胺 D3 受体结合的选择性高 900 倍(Ki 人类=1.7 nM,大鼠=0.84纳米)[7,8]。
体外活性:PD-128907 HCl 是一种有效的选择性多巴胺 D3 受体激动剂,EC50 为 0.64 nM,选择性是多巴胺 D2 受体的 53 倍。当使用[ 3 H]螺哌隆作为CHOK1细胞中的放射性配体时,PD-128907对人D3受体(Ki,1nM)表现出对人D2受体(Ki,1183nM)约1000倍的选择性,并且对人D2受体(Ki,1183nM)表现出约10000倍的选择性。人类 D4 受体(Ki,7000 nM)。 PD 128907 用于研究这些受体在大脑中的作用,例如限制多巴胺进一步释放的抑制性自身受体。激酶测定: (+)-PD 128907 Hydrochloride 是一种选择性多巴胺 D2/D3 受体激动剂,人和大鼠 D3 受体的 Kis 分别为 1.7、0.84 nM,人和大鼠 D3 受体的 Kis 分别为 179、770 nM。 在表达人D2或D3受体的转染细胞系中,通过掺入[3H]胸苷测量了一系列多巴胺激动剂增加有丝分裂的功能效力。激动剂的功能选择性与其结合选择性明显不同。(+)结合研究中D3受体选择性最强的化合物7-OH-DPAT、普拉克索、奎宁和PD-128907在功能测试中D3受体的效力分别是D2受体的7、15、21和54倍。溴隐亭对D2受体显示出10倍的功能选择性。D3选择性激动剂的已知行为支持D3受体在运动抑制中的作用,在多巴胺激动剂治疗运动功能障碍时应考虑到这一点。[1] PD-128907[4a R,10 b R-(+)-反式-3,4,4a,10 b-四氢-4-正丙基2H,5H-[1]苯并吡喃并[4,3-b]1,4-恶嗪-9-醇。],选择性多巴胺(DA)D3受体激动剂配体对人D3受体(Ki,1nM)的选择性约为人D2受体(Ki,1183nM)的1000倍,对使用[3H]螺珀酮作为CHO-K1细胞中的放射性配体的人D4受体(Ki,7000nM)的选择性为10000倍。[3H]PD-128907的研究表明,与CHO-K1细胞(CHO-K1-D3)中表达的人D3受体具有饱和、高亲和力的结合,平衡解离常数(Kd)为0.99 nM,结合密度(Bmax)为475 fmol/mg蛋白质。在相同条件下,表达人D2受体(CHO-K1-D2)的CHO-K1细胞没有明显的特异性结合。抑制[3H]PD 128907与参考DA试剂结合的效力的等级顺序与D3受体的报告值一致。这些结果表明,[3H]PD 128907是一种新的、高度选择性的D3受体配体,具有高比活性、高特异性结合和低非特异性结合,因此可用于进一步表征DA D3受体。[2] 本研究确定了PD 128907[R-(+)-反式-3,4,4a,10b-四氢-4-丙基-2H,5H-[1]苯并吡喃并[4,3-b]-1,4-恶嗪-9-ol]的生化和药理作用,这是一种多巴胺(DA)受体激动剂,对人类D3受体有偏好。在转染的中国仓鼠卵巢细胞(CHO K1)中,PD-128907以双相方式置换[3H]螺吡喃酮,这最适合双位点模型,D2L受体的高亲和力位点和低亲和力位点的Ki值分别为20和6964 nM,D3受体的相应位点分别为1.43和413 nM。向D2L和D3中添加钠和GTP类似物Gpp(NH)p会导致化合物亲和力的适度降低,这暗示了激动剂型作用。在激动剂结合([3H]N-0437)中,PD 128907对D3的选择性是D2L的18倍,与拮抗剂结合高亲和力位点时的选择性相似。PD-128907对D4.2受体仅表现出微弱的亲和力(Ki=169 nM)。未观察到对多种其他受体的显著亲和力。PD-128907在转染了D2L或D3受体的CHO p-5细胞中刺激细胞分裂(通过[3H]胸苷摄取测量),与D2L受体相比,其激活D3的效力高出约6.3倍[4]。 |
| 体内研究 (In Vivo) |
D3 敲除小鼠中 (+)-PD 128907 透析液 DA 水平显着降低。对于野生型和 D3 敲除小鼠,IC25 值分别为 61 nM 和 1327 nM。当将缺乏 D3 受体的小鼠与野生型透析液 DA 水平进行比较时,IC25 值的比率显示 (+)-PD 128907 在降低 DA 水平方面的作用强 22 倍。在野生型小鼠中,D3 激动剂以剂量相关的方式减少透析液 DA。根据事后分析,所有测试剂量(0.03、0.1 和 0.3 mg/kg)均显着抑制透析液 DA。在野生型和基因敲除小鼠的腹侧纹状体中,IC25值分别为0.05和0.44 mg/kg,表明全身施用(+)-PD 128907在降低透析液DA方面比D3有效9倍。消灭老鼠。在野生型和 D3 突变小鼠中,(+)-PD 128907 在剂量为 1 mg/kg 或以上时,透析液 DA 显着降低 [9]。
PD 128907 可有效减少正常大鼠和 γ-丁内酯 (GBL) 治疗大鼠的 DA 合成。 PD 128907(3 mg/kg)可降低可卡因过量的毒性,完全防止可卡因的惊厥和致命作用。这种保护通过与 D3 相关的机制发生,并且这种保护延伸到癫痫发作。 多巴胺(DA)自身受体沿着中脑DA神经元的躯体化程度表达,调节冲动活动,而在DA神经末梢表达的多巴胺自身受体则调节DA的合成和释放。大量证据表明,这些DA自身受体是DA受体的D2亚型。然而,许多药理学研究表明DA D3受体具有自身受体作用。这种可能性是通过基因靶向缺乏D3受体的小鼠进行测试的。D3受体突变体和野生型小鼠黑质和腹侧被盖区DA神经元的基础放电率没有差异。假定的D3受体选择性激动剂R(+)-反式-3,4,4a,10b-四氢-4-丙基-2H,5H-(1)苯并吡喃(4,3-b)-1,4-恶嗪+++-9-ol(PD-128907)在抑制两组小鼠中脑DA神经元的活性方面是等效的。在DA自身受体功能的γ-丁内酯(GBL)模型中,突变型和野生型小鼠在纹状体DA合成及其被PD-128907抑制方面是相同的。腹侧纹状体DA释放的体内微透析研究显示,突变小鼠的细胞外DA基础水平较高,但PD 128907对突变和野生型小鼠的抑制作用相似。这些结果表明,PD-128907对多巴胺细胞功能的影响反映了D2受体的刺激,而不是D3受体的刺激。尽管D3受体似乎没有显著参与DA自身受体功能,但它们可能参与DA释放的突触后激活短环反馈调节。[3] 在体内,该化合物在正常和γ-丁内酯(GBL)治疗的大鼠中均具有减少DA合成的活性;在GBL模型中,与D3受体表达较低的纹状体相比,D3表达较高的中脑边缘区的下降幅度更大。PD-128907降低了大鼠纹状体、伏隔核和内侧额叶皮层以及猴壳核中DA的释放(通过脑微透析测量)。从行为上讲,PD-128907在低剂量下降低了大鼠的自发运动活动(LMA),而在高剂量下观察到了刺激作用。高剂量的PD-128907与D1激动剂SKF 38393联合使用,逆转了利血平诱导的喉罩减少和诱导的立体型,表明了突触后DA激动剂的作用。目前尚不清楚DA受体的哪种亚型可能介导PD 128907的药理作用。然而,目前的研究结果表明,PD 128907显示出对DA D3的偏好,而不是D2L和D4.2受体,这表明它在低剂量下的作用可能是由于与D3受体的相互作用,在高剂量下,它可能与D2和D3受体都相互作用。[4] 可卡因滥用是一个公共卫生问题,过量服用会导致癫痫发作和死亡。在本研究中,多巴胺D(3/)D(2)受体激动剂剂量依赖性地完全预防了可卡因的惊厥和致死作用。优选D(3)的激动剂R-(+)-反式-3,4a,10b-四氢-4-丙基-2H,5H-[1]苯并吡喃并[4,3-b]-1,4-恶嗪-9-ol)[(+)-PD-128907],(+)-7-羟基二丙基氨基四氢萘,以及混合的D(3/)D(2)激动剂奎尼罗和奎洛烷都对小鼠可卡因毒性有效。这些化合物的抗惊厥作用发生在低于倒置筛查测试中评估的产生运动损伤的剂量时。(+)-PD 128907还赋予了对选择性多巴胺摄取抑制剂1-[2-[双(4-氟苯基)甲氧基]乙基]-4-[3-苯基-丙基]哌嗪(GBR 12909)惊厥作用的保护。(+)-PD-128907(3 mg/kg)对这些多巴胺能化合物的作用可能具有选择性,这可以通过其对通过其他神经机制起作用的多种惊厥药[戊四唑、(+)-荷包牡丹碱和苦味毒、4-氨基吡啶和叔丁基双甘膦酰硫代酯、N-甲基-d-天冬氨酸盐、红藻氨酸、毛果芸香碱、尼古丁、士的宁、氨茶碱、阈值电击和6-Hz电刺激]普遍缺乏保护作用来证明。直接和相关证据表明,这些作用是由D(3)受体介导的。D(3)受体拮抗剂[3-[4[1-(4-[2[4-(3-二乙氨基-丙氧基)-苯基]-苯并咪唑-l-基]-丁基)-1H-苯并咪唑-2-基]-苯氧基]-丙基)-二乙胺具有立体特异性和可逆性;PD 58491]但不包括D(2)受体[3[[4-(4-氯苯基)-4-羟基哌啶-1-基]甲基-1H-吲哚;L-741626。抗惊厥效力与D(3)受体功能测定中的效力呈正相关,但与D(2)受体功能无关。总之,这些发现表明,PD-128907预防可卡因抽搐和致死可能是由于D(3)受体介导的事件。[5] 先前的研究结果表明,多巴胺D(3)/D(2)受体激动剂在急性施用可卡因的惊厥和致死作用中具有保护作用。这里提供的数据表明,保护是通过D(3)连接的机制发生的,并且保护范围扩展到癫痫发作点燃。D(3)拮抗剂SB-277011-A[4-喹啉甲酰胺,N-[反式-4-[2-(6-氰基-3,4-二氢-2(1H)-异喹啉基)乙基]-环己基]-(9CI)]阻止了D(3”/D(2)受体激动剂(+)-PD-128907[(R-(+)-反式-3,4a,10b-四氢-4-丙基-2H,5H-[1]苯并吡喃并[4,3-b]-1,4-恶嗪-9-ol)]对可卡因诱导的癫痫发作的抗惊厥作用。D(3)/D(2)激动剂(+)-PD-128907提供的保护在D(3”受体缺陷小鼠中被消除。在D(2)受体敲除小鼠中,(+)-PD-128907的抗惊厥作用得以保留。(+)-PD-128907还可以预防因每天重复服用60mg/kg可卡因而引起的可卡因引发的癫痫发作。(+)-PD-128907也阻断了由可卡因引发的小鼠癫痫发作。尽管反复服用可卡因可以提高可卡因产生癫痫发作和致死的效力(ED(50)值降低),但每天联合服用(+)-PD-128907可以显著防止这种效力变化。在每天服用可卡因和(+)-PD-128907的小鼠中,D(3)受体的密度增加,但亲和力没有增加。(+)-PD-128907对这种可卡因驱动的过程起作用的特异性得到了证明,(+)-PD-128907对GABA(a)受体拮抗剂戊四唑的癫痫发作没有显著影响。结合文献研究结果,数据表明多巴胺D(3)受体在启动抑制可卡因毒性作用的机制中起作用,这一发现可能与药物依赖、精神分裂症和双相抑郁症等精神疾病有关[6]。 多巴胺D3受体在行为中的具体作用尚未阐明。我们现在报告说,多巴胺D2/D3激动剂在大鼠中引发剂量依赖性的打哈欠行为,导致倒U形剂量反应曲线。一系列实验旨在提出一种假设,即诱导打哈欠是D3受体介导的效应,而在较高剂量下观察到的打哈欠抑制是由于竞争性D2受体活性。我们比较了几种具有一系列体外D3选择性的多巴胺能激动剂,包括PD-128907[(S)-烷[(5aR反式)-5,5a,6,7,8,9,9a,10-八氢-6-丙基吡啶并[2,3-g]喹唑啉-2-胺二盐酸盐],普拉克索(N'-丙基-4,5,6,7-四氢苯并噻唑-2,6-二胺),7-OH-DPAT[(+/-)-7-羟基-2-二丙基氨基四氢萘-HBr],奎尼罗[反式-(-)-(4aR)-4,4a,5,6,7,8,18a,9-八氢-5-丙基-1H-吡唑并[3,4g]喹啉盐酸盐],溴隐亭[(+)-2-溴-12'-羟基-2'-(1-甲基乙基)-5'-(2-甲基丙基)麦角胺-3',6'-18-三酮甲磺酸盐]和阿扑吗啡[(R)-(-)-5,6,6a,7-四氢-6-甲基-4H-二苯并-[de,g]喹啉-10,11-二醇HCl]诱导大鼠打哈欠的能力。评估了一系列对D3的选择性不同于D2受体的D2/D3拮抗剂改变多巴胺激动剂作用的能力。拮抗剂L-741626(3-[4-(4-氯苯基)-4-羟基哌啶-L-基]甲基-1H-吲哚)、氟哌啶醇(4-[4-(4-氯苯)-4-羟基-1-哌啶基]-1-(4-氟苯基)-1-丁酮HCl)、萘呋啶(N-[(1-丁基-2-吡咯烷基)甲基]-4-氰基-1-甲氧基-2-萘甲酰胺)、U99194(2,3-二氢-5,6-二甲氧基-N,N-二丙基-1H-茚-2-胺马来酸酯)、SB-277011A(反式-N-[4-[2-(6-氰基-1,2,3,4-四氢异喹啉-2-基)乙基]环己基]-4-喹啉甲酰胺)和PG01037(N-{4-[4-(2,3-二氯苯基)-哌嗪-1-基]-反式-2-丁基}-4-吡啶-2-基苯甲酰胺-HCl)用于确定对D2/D3激动剂诱导的打哈欠的剂量反应曲线的影响。此外,使用一系列药理学工具研究了胆碱能和/或5-羟色胺能机制对打哈欠反应的潜在贡献,包括东莨菪碱[(a,S)-a-(羟甲基)苯乙酸(1a,2b,4b,5a,7b)-9-甲基-3-氧杂-9-氮杂三环[3.3.1.02,4]-壬基酯氢溴酸盐]、米安色林(1,2,3,4,10,14b-六氢-2-甲基二苯并[c,f]吡嗪并[1,2-a]氮杂环庚烷HCl)和D3偏好拮抗剂萘哌啶、U99194、SB-277011A和PG01037,以不同地调节PD诱导的打哈欠。-128907、毒扁豆碱[(3aS)-顺式-1,2,3,3a,8,8a-六氢-1,3a,8-三甲基吡咯并[2,3-b]吲哚-5-醇氨基甲酸甲酯半硫酸盐]和N-[3-(三氟甲基)苯基]哌嗪HCl。这些实验的结果提供了趋同的证据,表明多巴胺D2/D3激动剂诱导的打哈欠是D3激动剂介导的行为,随后对打哈欠的抑制是由竞争的D2激动剂活性驱动的。因此,多巴胺激动剂诱导的打哈欠可能代表了一种选择性鉴定D3和D2受体介导活性的体内方法。[7] 多巴胺D3受体的功能相关性尚未得到解决,主要是因为缺乏选择性D3受体配体。在本研究中,我们研究了(+)-PD 128907的体内特征,这是一种强效且功能选择性的D3受体激动剂。低剂量的(+)-PD-128907降低了大鼠的自发运动活动(ED50=13+/-3微克/千克,皮下注射),这一反应与非选择性D2,3受体激动剂阿扑吗啡(ED50=13+/-1.6微克/千克)相当。此外,(+)-PD 128907损害了对声惊吓反应的预脉冲抑制,当使用适当的预脉冲强度时,在30微克/千克的剂量下观察到显著影响。更高的剂量逆转了γ-丁内酯诱导的儿茶酚胺合成(伏隔核和纹状体的ED50=95+/-22和207+/-37微克/千克),并诱导了打哈欠(100-300微克/公斤)、阴茎梳理(30-1000微克/kg)和嗅闻(>=300微克/克),尽管需要比阿扑吗啡大3到10倍的剂量才能产生最大效果。然而,与阿扑吗啡相反,(+)-PD 128907未能在大鼠体内诱导强烈的刻板舔舐和叮咬。鉴于(+)-PD 128907对D3受体的效力和选择性,建议其在控制运动活动中发挥作用。此外,观察到(+)-PD 128907破坏了预脉冲抑制,这一现象在精神分裂症受试者中也受到损害,这可能表明这种受体亚型的病理重要性。[8] 药理学研究表明,D3多巴胺受体参与了细胞外多巴胺浓度的调节。然而,最近使用D3受体敲除小鼠的研究表明,以前归因于D3受体的几种功能是由其他受体类型介导的。在本研究中,我们使用无净通量微透析技术来表征:(i)D3敲除和野生型小鼠腹侧纹状体中的基础多巴胺动力学,以及(ii)推定的D3受体选择性激动剂(+)-PD-128907的影响。D3敲除小鼠和野生型小鼠的细胞外多巴胺浓度和体内提取分数(基础多巴胺摄取的间接指标)均无差异。此外,在两种基因型之间没有检测到钾(60 mM)或可卡因(5或20 mg/kg i.p.)诱发的多巴胺浓度的差异。然而,纹状体内或全身给药(+)-PD 128907剂量未能改变敲除小鼠的多巴胺浓度,显著降低了野生型小鼠的透析液多巴胺浓度。(+)-PD 128907的浓度反应曲线比较显示,在纹状体内输注后,野生型和敲除型小鼠的IC(25)值分别为61和1327 nM。在全身施用D3偏好激动剂后,也获得了类似的差异(IC(25)在野生型和敲除小鼠中分别为0.05和0.44mg/kg腹腔注射)。我们得出结论,D3受体的激活降低了细胞外多巴胺水平,在足够低的剂量下,(+)-PD 128907对细胞外多巴胺的影响是由D3受体选择性介导的[9]。 |
| 酶活实验 |
唇缘结合试验。[2]
测定条件如下所述。使用不同的Tris-HCl缓冲液进行了一系列平衡饱和度研究。简而言之,将50 pl的['Humigand(1 nM,竞争研究中的最终浓度)、50 pl的药物或缓冲液以及400 pl的脑或CHO Kl细胞膜加入到适当的冰冷缓冲液中,使总体积为500 l_l。在25℃下孵育60分钟,然后通过Whatman GF/B玻璃纤维过滤器在Brandel MR48细胞采集器上用1 ml缓冲液洗涤四次(在0.5%PEI中预浸约一小时)来终止孵育。加入10 ml液体闪烁Ready Gel鸡尾酒并提取过夜后,用Beckman LS 6800液体计数过滤器上剩余的放射性。闪烁计数器(50%效率)。特异性结合定义为在1pM氟哌啶醇存在下的总结合减去结合,范围为90-95%。所有检测均一式三份。CHOKl细胞的粗膜每个测定管的蛋白质含量在40pg-60pg之间。蛋白质通过Bradford测定法使用酶标仪进行分析。 饱和度研究。[2] 用九种浓度递增的[w]PD-128907(0.039-10.0 r&l)测定饱和结合曲线。如前所述,在25℃下用组织匀浆孵育60分钟。通过使用不同的缓冲液(TE=25 mM Tris-HCl,1 mM EDTA;TEM=25 mM Tris-HCl,1 mM乙二胺四乙酸,6 mM氯化镁,TEN=2.5 mM Tris-HHCl,1 mmol EDTA,12 动力学实验。[2] 使用TEM缓冲液用['H]PD-128907(1.0 nM,终浓度)进行缔合和解离实验。在关联实验中,在1pM氟哌啶醇存在下定义特异性结合,并在不同时间过滤样品。在解离研究中,还通过添加过量的PD-128907(最终浓度为20 pM)在不同时间(按照上述确定的平衡度)过滤样品。离解和缔合的速率常数之比(k-l/k+l)用于计算r3H]PD 128907(n=3)的&。 共代谢研究。[2] 在CHO-Kl-D中使用1 nM[SH]PD-128907测定各种药物的抑制常数(IS,),细胞膜和测定用TEM缓冲液进行。制备了八种不同浓度的每种配体,并进行了三次研究。研究了非水解GTP类似物Gpp(NH)p对DA抑制[“H]PD 128907与CHO-Kl-D膜结合的影响。 (+)-PD 128907 盐酸盐是 D2/D3 多巴胺受体的选择性激动剂。其对人和大鼠 D3 受体的 Kis 值分别为 1.7、0.84 nM 和 179、770 nM。 |
| 细胞实验 |
组织培养。[2]
CHO细胞以及随后用人D、和D cDNA转染的CHO细胞在37℃的95%空气和5%CO的气氛下维持。CHO细胞在含有100 U/ml青霉素-链霉素和透析胎牛血清的F-12培养基中生长和传代。每2-3天更换一次培养基,在收获细胞前至少18小时更换一次。通过用含有0.05%EDTA的冷磷酸盐缓冲盐水(PBS)代替培养基,然后在1000 g下离心2分钟,收获融合的培养物。将获得的颗粒悬浮在适当体积的冰冷缓冲液(25 mM Tris-HCl,1 mM EDTA,pH 7.4,TE缓冲液)中,并在4℃下在20000 g下离心15分钟。将得到的最终颗粒悬浮在TE缓冲液中,在6℃下用Polytron均质化5秒,用于放射性配体结合测定,或储存在-80*C下。将用于测定的CHO Kl细胞膜在6℃用Polytron。 |
| 动物实验 |
Extracellular single-unit recordings. [3]
All methods for extracellular single-cell recordings were similar to those previously reported (White et al., 1995), although modified somewhat for the mouse (Xu et al., 1994). Briefly, mice were anesthetized with chloral hydrate (400 mg/kg, i.p.) and mounted in a standard stereotaxic apparatus with a specialized adapter for the mouse. Body temperature was maintained at 36–37.5°C with a thermostatically controlled heating pad. A 28 gauge (3/8 inch) hypodermic needle was placed in a lateral tail vein through which additional anesthetic (as required) and drugs of study were administered. A burr hole was drilled in the skull, and the dura was retracted from the area overlying the VTA and SN, 0.4–1.3 mm anterior to lambda and 0.2–1.0 mm lateral to the midline. Recording electrodes were made by pulling glass tubing [outer diameter (o.d.), 2.0 mm], which was prefilled with fiberglass, and by breaking the tip back to a diameter of 1–2 μm. Electrodes were filled with 2 m NaCl saturated with 1% (w/v) fast green dye and typically exhibitedin vitro impedances of 1–3 MΩ (at 135 Hz). Electrode potentials were passed through a high impedance amplifier/filter and displayed on an oscilloscope. Individual action potentials were discriminated electronically and monitored with an audio amplifier. Integrated rate histograms, generated by the output of the window discriminator, were plotted by a polygraph recorder, whereas digitized counts of cellular activity were obtained for off-line analysis. Electrodes were lowered to a point 0.5 mm above the VTA and SN and then slowly advanced with a hydraulic microdrive through the DA cell regions (3.2–5.0 mm ventral to the cortical surface). DA cells were identified by standard physiological criteria (Bunney et al., 1973; Wang, 1981;Sanghera et al., 1984) and were recorded for 3–6 min to establish a baseline firing rate. To determine the sensitivity of impulse-modulating somatodendritic autoreceptors, we administered PD-128907 to each mouse through the tail vein, using a cumulative dose regimen in which each dose doubled the previous dose, at 60–90 sec intervals. After agonist-induced inhibition, the D2-class receptor antagonist eticlopride was administered (0.1–0.2 mg/kg) to reverse the effect and confirm receptor mediation. At the end of each experiment, the cell location was marked by ejecting fast green dye, and the spot was verified by routine histological assessment (described below). γ-Butyrolactone experiments. [3] The l-aromatic amino acid decarboxylase inhibitor NSD 1015 was administered 30 min before death (100 mg/kg, i.p.). γ-Butyrolactone (GBL) was administered (750 mg/kg, i.p.) 5 min before NSD 1015 to eliminate impulse flow in DA neurons (Walters and Roth, 1976). PD-128907 was injected 5 min before GBL. Mice were killed by decapitation, and the brains were quickly removed. Dorsal and ventral striatum were dissected on a chilled glass plate with the aid of a mouse brain matrix designed to allow coronal sections to be cut rapidly and reproducibly (Activational Systems, Warren, MI). Two slices (2 mm each) were taken, beginning at the rostral boundary of the olfactory tubercle. The most anterior slice was the source of ventral striatum, whereas both slices were used to obtain dorsal striatum. The ventral striatum was dissected with angular cuts originating at the lateral olfactory tracts and ending at the midline (average weight, 18.5 mg). The remainder of the striatal region from that slice as well as the similar region from the more caudal slice was considered the dorsal striatum (average weight, 35.8 mg). Tissues were kept at −80°C. To measure tissue catechols, we weighed frozen tissues and then sonically disrupted them in a homogenization solution consisting of 100 mmHClO4, 5 mmNa2S2O5, and α-methyl dopa, an internal standard. After centrifugation (25,000 ×g for 10 min), aliquots of the supernatant were processed by alumina extraction as described previously (Galloway et al., 1986). The 3,4-dihydroxyphenylalanine (DOPA) content of samples was determined using an HPLC system consisting of a Bioanalytical Systems (BAS) Phase II ODS 3 μm column (100 × 3.2 mm), a BAS LC4C electrochemical detector, and a Scientific Systems model 222C HPLC pump. The mobile phase consisted of 0.1 mNaH2PO4, 1 mm EDTA, 0.2 mm 1-octane-sulfonic acid, and 3% methanol, adjusted to pH 2.7 with phosphoric acid. DOPA content was quantified based on both internal and external standards. In vivo microdialysis experiments. [3] Mice used for dialysis experiments weighed 28–35 gm. Concentric microdialysis probes were constructed as described previously, with fused-silica inlet and outlet lines (Wolf et al., 1994). Dialysis membrane (molecular weight cutoff, 6000; o.d., 250 μm) was obtained from Spectrum (Los Angeles, CA). Data were not corrected for in vitro probe recovery because probes may suffer differential alterations during insertion. Consistent with this assumption, data sets corrected for recovery often exhibit greater variability than do those that are uncorrected (Xue et al., 1996). Probes were stereotaxically implanted under sodium Brevital (8 mg/kg, i.p.). Stereotaxic coordinates were, relative to bregma, anterior, 1.5 mm; lateral, 1.5 mm; and ventral, 2.7–4.7 mm. Ventral coordinates indicate exposed regions of dialysis membrane (2 mm). After surgery, mice were placed in Plexiglas cages (23 × 46 mm) and allowed to recover overnight. Food and water were available ad libitum. Dialysis cages were equipped with balance arms (Instech, Plymouth Meeting, PA), and homemade liquid swivels and tethers were constructed from plastic syringes and tubing. These were used because commercially available swivels were too heavy for use with mice. Probes were perfused overnight at 0.3 μl/min with artificial CSF (aCSF) consisting of (in mm): 2.7 KCl, 140 NaCl, 1.2 CaCl2, 1 MgCl2, 0.3 NaH2PO4, and 1.7 Na2HPO4, pH 7.4. The next morning, the perfusion rate was increased to 2 μl/min for 2–3 hr before the experiment was begun. Experiments consisted of 1 hr of perfusion with control aCSF to determine basal DA efflux, 1 hr of perfusion with aCSF containing PD-128907, and a 1 hr recovery period during which control aCSF was perfused. Thus, administration of PD-128907 occurred ∼20 hr after probe implantation. Fractions were collected every 20 min. After each experiment, mice were anesthetized and perfused intracardially with normal saline followed by 10% formalin. Probe placement was examined in sections stained with cresyl violet. Only data from mice with verified probe placements were included in the analysis. Dialysates were analyzed for DA content using the HPLC system described for GBL experiments. Chromatographic conditions were optimized for early elution of DA to obtain maximum sensitivity. The mobile phase consisted of 0.1 m NaH2PO4, 0.5 mm EDTA, 2 mm 1-octane-sulfonic acid, and 16% methanol, adjusted to pH 4.9. Peaks were recorded using a dual-channel chart recorder and were quantified by comparison with the peak heights of external standards run with every experiment. |
| 参考文献 |
[1]. Neuroreport. 1995 Jan 26;6(2):329-32. [2]. Life Sci. 1995;57(15):1401-10. [3]. J Neurosci. 1998 Mar 15;18(6):2231-8. [4]. J Pharmacol Exp Ther. 1995 Dec;275(3):1355-66. [5]. J Pharmacol Exp Ther. 2004 Mar;308(3):957-64. [6]. J Pharmacol Exp Ther. 2008 Sep;326(3):930-8. [7]. J Pharmacol Exp Ther. 2005 Jul;314(1):310-9. |
| 其他信息 |
As mentioned above, studies conductedwith [SH]PD-128907 in CHO-Kl-D, membranes in the presence of Na’ (120 mM) showed that the K, was slightly lower, although not statistically significant. These effects may indicate that Na’is not inducing a conformational change in the D, receptor as has previously been suggested for D, receptors. Besides Na’, guanyl nucleotides have also been shown to decrease the binding of agonists to G-protein linked receptors. Inclusion of 100 PM of the nonhydrolyzable GTP analog, Gpp(NH)p in the DA displacement curves did not alter the competition curves for DA, indicating a lack of any significant functional coupling of the human D, receptor to a G-protein regulated signal transduction system in CHO-Kl cells (Table II). These findings are in agreement with previously published findings in CHO and MN9D cells indicating a lack of or only weak coupling of D, receptors to G-proteins (see ref. 2.5 and 26 for opposite results). However, another study has shown that recombinant D,receptors heteregously expressed in CHO cells functionally interact with endogenous G proteins. The reasons for these differences not clear but may be due to different signalling pathways in certain clone of the cells used where G protein modulation have been described. In agreement with our present data, weak or no modulatory effects of guanyl nucleotide on DA binding at native D,receptors were also observed using [SH]7-OH-DPAT and [‘*‘1]7-OH-PIPAT, respectively. The absence of appropriate subtypes of G proteins in the various cell lines and/or the D, receptor are possible explanations for the phenomenon if the natural coupling mechanism/channel is not present or is not G-protein linked. [‘H]PD-128907 appears to be a very selective D,radioligand with high specific activity, good specific yield and low non-specific binding. [3H]PD 128907 shoud be appropriate for such studies as autoradiography to evaluate the presence and distribution of D, receptors in brain and possibly to characterize the function, if any, of this receptor subtype. [2]
Our microdialysis studies indicated a significant increase in basal DA efflux in the ventral striatum of D3 receptor mutant mice compared with their wild-type littermates. Although “no-net flux” dialysis studies are often needed to quantify differences in basal transmitter efflux in vivo (for review, see Justice, 1993), the difference between D3 receptor mutant and wild-type mice was quite robust, with all but one mutant mouse showing higher basal DA efflux than any of the wild-type mice. Increased basal DA efflux might be expected if nerve terminal D3 autoreceptors normally exert a tonic inhibitory influence on DA release. However, the results obtained with PD 128907 are incompatible with this simple interpretation because this putative D3 receptor-selective agonist inhibited DA release by the same absolute amount in D3 receptor mutant and wild-type mice. Thus, no reduction of DA autoreceptor modulation occurred as a result of the D3 receptor mutation. Although the percent decrease caused by PD-128907 was smaller in the mutant mice, this effect was attributable to their higher basal DA levels, a factor known to reduce the ability of DA agonists to suppress DA release (Cubeddu and Hoffman, 1982; Dwoskin and Zahniser, 1986; for review, see Wolf and Roth, 1987). An alternative explanation for our findings is that DA release is normally modulated both by D2 release-modulating autoreceptors and by negative feedback pathways engaged by postsynaptic D3 (and other D2-class) receptors. Loss of D3 receptor-mediated inhibitory feedback would explain increased basal DA efflux, whereas activation of D2release-modulating autoreceptors by PD-128907 would explain the normal inhibitory effects on DA release. This model is consistent with the D3 mRNA findings indicating that D3 receptors are distributed primarily within target neurons of the ascending DA systems. In ventral striatal terminal fields where the D3receptor is most highly expressed (nucleus accumbens shell, olfactory tubercle, and islands of Calleja), there is good agreement between levels of D3 receptor mRNA and D3 receptor binding sites (Diaz et al., 1995), suggesting that most D3receptors exist on dendrites, soma, or local terminals of intrinsic neurons. If postsynaptic D3 receptors are coupled to feedback pathways that normally exert an inhibitory influence on DA release, the loss of such feedback might lead to increased basal DA efflux but leave D2 autoreceptor-mediated effects intact.[3] |
| 分子式 |
C14H19NO3
|
|---|---|
| 分子量 |
249.30556
|
| 精确质量 |
285.113
|
| 元素分析 |
C, 58.84; H, 7.05; Cl, 12.41; N, 4.90; O, 16.80
|
| CAS号 |
300576-59-4
|
| 相关CAS号 |
23594-64-9;300576-59-4 (HCl); 112960-16-4;
|
| PubChem CID |
11957668
|
| 外观&性状 |
White to off-white solid powder
|
| 沸点 |
389ºC at 760mmHg
|
| 闪点 |
189ºC
|
| LogP |
2.676
|
| tPSA |
41.93
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
19
|
| 分子复杂度/Complexity |
286
|
| 定义原子立体中心数目 |
2
|
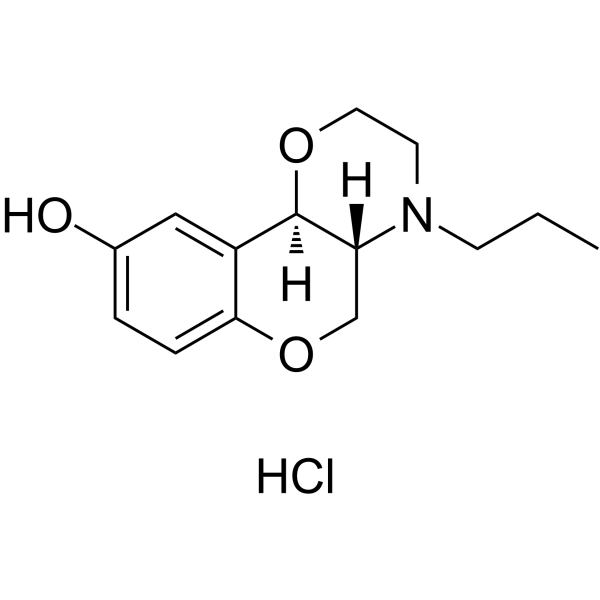
| SMILES |
CCCN1[C@@]2([H])[C@@](OCC1)([H])C3=CC(O)=CC=C3OC2.Cl
|
| InChi Key |
DCFXOTRONMKUJB-QMDUSEKHSA-N
|
| InChi Code |
InChI=1S/C14H19NO3.ClH/c1-2-5-15-6-7-17-14-11-8-10(16)3-4-13(11)18-9-12(14)15;/h3-4,8,12,14,16H,2,5-7,9H2,1H3;1H/t12-,14-;/m1./s1
|
| 化学名 |
(4aR,10bR)-4-propyl-3,4a,5,10b-tetrahydro-2H-chromeno[4,3-b][1,4]oxazin-9-ol;hydrochloride
|
| 别名 |
112960-16-4; 300576-59-4; (+)-PD 128907 hydrochloride; (4aR,10bR)-rel-4-Propyl-2,3,4,4a,5,10b-hexahydrochromeno[4,3-b][1,4]oxazin-9-ol hydrochloride; (+/-)-PD 128,907 hydrochloride; PD128907 Hydrochloride; S(+)-PD 128,907 hydrochloride; PD128907 HCl;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~20.83 mg/mL (~72.89 mM)
H2O : ~16.67 mg/mL (~58.33 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (7.28 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (7.28 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (7.28 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.0111 mL | 20.0554 mL | 40.1107 mL | |
| 5 mM | 0.8022 mL | 4.0111 mL | 8.0221 mL | |
| 10 mM | 0.4011 mL | 2.0055 mL | 4.0111 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。