| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
(R)-(-)-Phenylephrine 对 α1D、α1B 和 α1A 受体的 pKi 值分别为 5.86、4.87 和 4.70,使其成为选择性 α1-肾上腺素能受体激动剂 [1][2]。这种心肌可能是心肌纤维化的治疗目标,因为去氧肾上腺素刺激心脏成纤维细胞,表明Ca (2+)/CaN/NFAT 不会驱动去氧肾上腺素诱导的心脏成纤维细胞增殖[3]。
|
|---|---|
| 体内研究 (In Vivo) |
用 100 μM 去氧肾上腺素灌注心脏后,两种 p38-MAPK 亚型均快速激活 12 倍(最多 10 分钟)。 α1-Syntropin 对增强心脏收缩力的麻醉剂(包括去氧肾上腺素)有反应。新生儿心室肌细胞的 SAPK 和 JNK 被去氧肾上腺素激活[4]。去氧肾上腺素具有加速肺水肿吸收、提高气量通气运输的肺泡液清除率的能力[5]。
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Phenylephrine is 38% orally bioavailable. Clinically significant systemic absorption of ophthalmic formulations is possible, especially at higher strengths and when the cornea is damaged. 86% of a dose of phenylephrine is recovered in the urine with 16% as the unmetabolized drug, 57% as the inactive meta-hydroxymendelic acid, and 8% as inactive sulfate conjugates. The volume of distribution of phenylephrine is 340L. Phenylephrine has an average clearance of 2100mL/min. Phenylephrine undergoes rapid distribution into peripheral tissues; there is some evidence that the drug may be stored in certain organ compartments. The pharmacologic effects of phenylephrine are terminated at least partially by uptake of the drug into tissues. Penetration of phenylephrine into the brain appears to be minimal. Phenylephrine does not appear to be distributed to any great extent into breast milk. Phenylephrine is completely absorbed following oral administration and undergoes extensive first-pass metabolism in the intestinal wall. The bioavailability of phenylephrine following oral administration is approximately 38% relative to IV administration. Because of extensive first-pass metabolism, there is considerable interindividual and possibly intraindividual variation in oral bioavailability of the drug. Following oral administration of phenylephrine (1 or 7.8 mg), peak serum concentrations occur at 0.75-2 hours. Phenylephrine and its metabolites are excreted mainly in urine. Following oral or IV administration, approximately 80 or 86% of the dose, respectively, is excreted in urine within 48 hours, principally as metabolites; approximately 2.6% of an oral dose or 16% of an IV dose is excreted in urine as unchanged drug. 7-3H-phenylephrine was given to 15 volunteers by a short-infusion n = 4) or p.o. (10 volunteers, 1 patient with porto-caval anastomosis). Analysis of serum for free 3H-phenylephrine and fractionation of urinary radioactivity was performed by ion-exchange and thin-layer chromatography. As almost the same 3H-activity was excreted in urine after i.v. and p.o. administration, 86% and 80% of the dose respectively, complete enteral absorption can be assumed. A considerable difference was seen in the fraction of free phenylephrine, i.v. 16% of the dose versus p.o. 2.6%, which suggested reduced bioavailability. This was confirmed by comparison of the areas under the serum curve, which showed a bioavailability factor of 0.38. The result for the patient with porto-caval anastomosis was comparable to that in the normal volunteers. The biological half-life of 2 to 3hr was comparable to that of structurally related amines, as were the total clearance of 2 L/hr, and the volume of distribution of 340 L. Metabolism / Metabolites Phenylephrine is mainly metabolized by monoamine oxidase A, monoamine oxidase B, and SULT1A3. The major metabolite is the inactive meta-hydroxymandelic acid, followed by sulfate conjugates. Phenylephrine can also be metabolized to phenylephrine glucuronide. Phenylephrine undergoes extensive metabolism in the intestinal wall (first-pass) and in the liver. The principal routes of metabolism involve sulfate conjugation (primarily in the intestinal wall) and oxidative deamination (by monoamine oxidase (MAO)); glucuronidation also occurs to a lesser extent. 7-3H-phenylephrine was given to 15 volunteers by a short-infusion n = 4) or p.o. (10 volunteers, 1 patient with porto-caval anastomosis). Analysis of serum for free 3H-phenylephrine and fractionation of urinary radioactivity was performed by ion-exchange and thin-layer chromatography. ... Metabolism to phenolic conjugates mainly after oral ingestion, and to m-hydroxymandelic acid after i.v. injection, again demonstrated that m-hydroxylated amines are predominantly conjugated during the "first-pass" metabolism. Phenylephrine has known human metabolites that include (2S,3S,4S,5R)-3,4,5-trihydroxy-6-[3-[(1R)-1-hydroxy-2-(methylamino)ethyl]phenoxy]oxane-2-carboxylic acid. Biological Half-Life Intravenous phenylephrine has an effective half life of 5 minutes and an elimination half life of 2.5 hours. The elimination half-life of phenylephrine averages 2-3 hours following oral or IV administration |
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
Administration of a 10% solution of phenylephrine hydrochloride to patients pretreated with 2% pilocarpine hydrochloride produces mydriasis but to a lesser degree than occurs in patients who are not receiving the miotic. Pilocarpine may prevent or reduce visual disturbances and the risk of increased intraocular pressure associated with mydriasis in some patients and may be used to hasten recovery from mydriasis after ophthalmologic examination. Phenylephrine may reduce ciliary and conjunctival congestion and accommodative myopia often encountered when miotics are used alone in the treatment of glaucoma, without compromising the effectiveness of glaucoma therapy. Concomitant administration of phenylephrine with cycloplegic antimuscarinic drugs such as atropine sulfate, cyclopentolate hydrochloride, homatropine hydrobromide, or scopolamine hydrobromide produces increased dilation of the pupil which is of clinical value. The possibility that digitalis can sensitize the myocardium to the effects of sympathomimetic drugs should be considered. The cardiac and pressor effects of phenylephrine are potentiated by prior administration of monoamine oxidase (MAO) inhibitors because the metabolism of phenylephrine is reduced. The potentiation is greater following oral administration of phenylephrine than after parenteral administration of the drug because reduction of the metabolism of phenylephrine in the intestine results in increased absorption of the drug. Oral administration of phenylephrine to patients receiving a MAO inhibitor should be avoided. Parenteral administration of phenylephrine to these patients, if unavoidable, should be undertaken with extreme caution and initial doses should be small. Patients should consult a clinician before initiating anorectal phenylephrine therapy if they are receiving an MAO inhibitor. For more Interactions (Complete) data for PHENYLEPHRINE (12 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
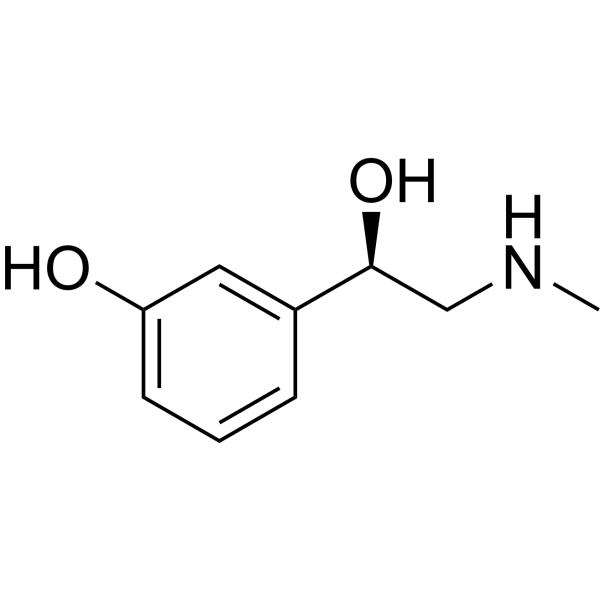
Phenylephrine is a member of the class of the class of phenylethanolamines that is (1R)-2-(methylamino)-1-phenylethan-1-ol carrying an additional hydroxy substituent at position 3 on the phenyl ring. It has a role as an alpha-adrenergic agonist, a cardiotonic drug, a mydriatic agent, a protective agent, a vasoconstrictor agent, a sympathomimetic agent and a nasal decongestant. It is a member of phenylethanolamines, a secondary amino compound and a member of phenols. It is a conjugate base of a phenylephrine(1+).
Phenylephrine is an alpha-1 adrenergic receptor agonist used to treat hypotension, dilate the pupil, and induce local vasoconstriction. The action of phenylephrine, or neo-synephrine, was first described in literature in the 1930s. Phenylephrine was granted FDA approval in 1939. Phenylephrine is an alpha-1 Adrenergic Agonist. The mechanism of action of phenylephrine is as an Adrenergic alpha1-Agonist. Phenylephrine is a direct-acting sympathomimetic amine chemically related to adrenaline and ephedrine with potent vasoconstrictor property. Phenylephrine is a post-synaptic alpha-adrenergic receptor agonist that causes vasoconstriction, increases systolic/diastolic pressures, reflex bradycardia, and stroke output. An alpha-1 adrenergic agonist used as a mydriatic, nasal decongestant, and cardiotonic agent. See also: Phenylephrine Hydrochloride (has salt form); Lidocaine; phenylephrine (component of) ... View More ... Drug Indication Phenylephrine is available in various drug formulations, which have different indications. Phenylephrine injections are indicated to treat hypotension caused by shock or anesthesia. The ophthalmic formulation is indicated to induce mydriasis and conjunctival vasoconstriction. The intranasal formulation is used to treat congestion, and a topical formulation is used to treat hemorrhoids. Off-label uses include priapism and induction of local vasoconstriction. Mechanism of Action Phenylephrine is an alpha-1 adrenergic agonist that mediates vasoconstriction and mydriasis depending on the route and location of administration. Systemic exposure to phenylephrine also leads to agonism of alpha-1 adrenergic receptors, raising systolic and diastolic pressure as well as peripheral vascular resistance. Increased blood pressure stimulates the vagus nerve, causing reflex bradycardia. Phenylephrine acts predominantly by a direct effect on alpha-adrenergic receptors. In therapeutic doses, the drug has no substantial stimulant effect on the beta-adrenergic receptors of the heart (beta1-adrenergic receptors) but substantial activation of these receptors may occur when larger doses are given. Phenylephrine does not stimulate beta-adrenergic receptors of the bronchi or peripheral blood vessels (beta2-adrenergic receptors). It is believed that alpha-adrenergic effects result from the inhibition of the production of cyclic adenosine-3',5'-monophosphate (cAMP) by inhibition of the enzyme adenyl cyclase, whereas beta-adrenergic effects result from stimulation of adenyl cyclase activity. Phenylephrine also has an indirect effect by releasing norepinephrine from its storage sites. Therapeutic Uses Adrenergic alpha-Agonists; Cardiotonic Agents; Mydriatics; Nasal Decongestants; Sympathomimetics; Vasoconstrictor Agents Nasal phenylephrine is indicated for the symptomatic relief of nasal congestion due to the common cold or hay fever, sinusitis, or other upper respiratory allergies. /Included in US product labeling/ Nasal phenylephrine may be useful in the adjunctive therapy of middle ear infections by decreasing congestion around the eustachian ostia. /Included in US product labeling/ Nasal phenylephrine is used for relief of sinus congestion. /NOT included in US product labeling/ For more Therapeutic Uses (Complete) data for PHENYLEPHRINE (18 total), please visit the HSDB record page. Drug Warnings Because it is not known whether phenylephrine is distributed into milk, the drug should be used with caution in nursing women. Administration of phenylephrine to patients in late pregnancy or labor may cause fetal anoxia and bradycardia by increasing contractility of the uterus and decreasing uterine blood flow. ... It is also not known whether the drug can cause fetal harm when administered to pregnant women. Phenylephrine should be used during pregnancy only when clearly needed. Ophthalmic use of phenylephrine occasionally causes systemic sympathomimetic effects such as palpitation, tachycardia, premature ventricular contractions, occipital headache, pallor or blanching, trembling or tremors, increased perspiration, and hypertension. In one patient, hypertension severe enough to cause subarachnoid hemorrhage followed insertion of a cotton wick saturated with 10% phenylephrine hydrochloride in the lower conjunctival cul-de-sac. ... Systemic effects occur only rarely after topical application of solutions containing 2.5% or less of phenylephrine hydrochloride to the conjunctiva but are more likely to occur if the drug is instilled after the corneal epithelium has been damaged (e.g., by trauma or instrumentation) or permeability is increased by tonometry, inflammation, surgery of the eye or adnexa, or topical application of a local anesthetic; when the eye or adnexa are diseased; or when lacrimation is suppressed such as during anesthesia. The risk of severe hypertension is greatest in infants receiving instillations of 10% phenylephrine hydrochloride solutions. In patients with shock, pressor therapy is not a substitute for replacement of blood, plasma, fluids, and/or electrolytes. Blood volume depletion should be corrected as fully as possible before phenylephrine is administered. In an emergency, the drug may be used as an adjunct to fluid volume replacement or as a temporary supportive measure to maintain coronary and cerebral artery perfusion until volume replacement therapy can be completed, but phenylephrine must not be used as sole therapy in hypovolemic patients. Additional volume replacement also may be required during or after therapy with the drug, especially if hypotension recurs. Monitoring of central venous pressure or left ventricular filling pressure may be helpful in detecting and treating hypovolemia; in addition, monitoring of central venous or pulmonary arterial diastolic pressure is necessary to avoid overloading the cardiovascular system and precipitating congestive heart failure. Hypoxia and acidosis, which also may reduce the effectiveness of phenylephrine, must be identified and corrected prior to or concurrently with administration of the drug. For more Drug Warnings (Complete) data for PHENYLEPHRINE (24 total), please visit the HSDB record page. Pharmacodynamics Phenylephrine is an alpha-1 adrenergic agonist that raises blood pressure, dilates the pupils, and causes local vasoconstriction. Ophthalmic formulations of phenylephrine act for 3-8 hours while intravenous solutions have an effective half life of 5 minutes and an elimination half life of 2.5 hours. Patients taking ophthalmic formulations of phenylephrine should be counselled about the risk of arrhythmia, hypertension, and rebound miosis. Patients taking an intravenous formulation should be counselled regarding the risk of bradycardia, allergic reactions, extravasation causing necrosis or tissue sloughing, and the concomitant use of oxytocic drugs. |
| 分子式 |
C9H13NO2
|
|---|---|
| 分子量 |
167.2
|
| 精确质量 |
167.095
|
| CAS号 |
59-42-7
|
| 相关CAS号 |
Phenylephrine hydrochloride;61-76-7
|
| PubChem CID |
6041
|
| 外观&性状 |
White to light yellow solid powder
|
| 密度 |
1.159 g/cm3
|
| 沸点 |
341.1ºC at 760 mmHg
|
| 熔点 |
171°C
|
| 闪点 |
163.4ºC
|
| 折射率 |
-55.5 ° (C=5, 1mol/L HCl)
|
| LogP |
1.035
|
| tPSA |
52.49
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
12
|
| 分子复杂度/Complexity |
130
|
| 定义原子立体中心数目 |
1
|
| SMILES |
CNC[C@@H](C1=CC(=CC=C1)O)O
|
| InChi Key |
SONNWYBIRXJNDC-VIFPVBQESA-N
|
| InChi Code |
InChI=1S/C9H13NO2/c1-10-6-9(12)7-3-2-4-8(11)5-7/h2-5,9-12H,6H2,1H3/t9-/m0/s1
|
| 化学名 |
3-[(1R)-1-hydroxy-2-(methylamino)ethyl]phenol
|
| 别名 |
Metasynephrine; Metaoxedrin; Phenylephrine
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~50 mg/mL (~299.03 mM)
H2O : ~5 mg/mL (~29.90 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 10 mg/mL (59.81 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 通过加热和超声助溶。
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.9809 mL | 29.9043 mL | 59.8086 mL | |
| 5 mM | 1.1962 mL | 5.9809 mL | 11.9617 mL | |
| 10 mM | 0.5981 mL | 2.9904 mL | 5.9809 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Blood PREssure Augmentation in Large-vessel Occlusion Stroke Study
CTID: NCT04218773
PhaseEarly Phase 1 Status: Enrollin
Phenylephrine versus EPhedrine on cerebral Perfusion during carotid EndarteRectomy: PEPPER study
CTID: null
Phase: Phase 4 Status: Completed
Date: 2012-07-30