| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
| 靶点 |
CFTR/cystic fibrosis transmembrane conductance regulator
|
|---|---|
| 体内研究 (In Vivo) |
Olacaftor(VX-440)是一种下一代校正器,在一项2期试验中进行了评估,该试验是随机、双盲、安慰剂和活性对照研究,旨在评估VX-440与特扎卡福和伊伐卡福三联使用在CF患者中的安全性和耐受性,这些CF患者是F508del突变和MF CFTR突变的杂合子,不太可能对特扎卡佛和/或伊伐卡佛治疗(F508del MF)有反应,或者是F508del突变的纯合子(ClinicalTrials.gov标识符:NCT02951195)[https://pmc.ncbi.nlm.nih.gov/articles/PMC7088950/].
|
| 参考文献 | |
| 其他信息 |
Background: Cystic fibrosis (CF) is a common life-shortening genetic condition caused by a variant in the cystic fibrosis transmembrane conductance regulator (CFTR) protein. A class II CFTR variant F508del is the commonest CF-causing variant (found in up to 90% of people with CF (pwCF)). The F508del variant lacks meaningful CFTR function - faulty protein is degraded before reaching the cell membrane, where it needs to be to effect transepithelial salt transport. Corrective therapy could benefit many pwCF. This review evaluates single correctors (monotherapy) and any combination of correctors (most commonly lumacaftor, tezacaftor, elexacaftor, VX-659, Olacaftor (VX-440) or VX-152) and a potentiator (e.g. ivacaftor) (dual and triple therapies).
Objectives: To evaluate the effects of CFTR correctors (with or without potentiators) on clinically important benefits and harms in pwCF of any age with class II CFTR mutations (most commonly F508del). Search methods: We searched the Cochrane CF Trials Register (28 November 2022), reference lists of relevant articles and online trials registries (3 December 2022). Selection criteria: Randomised controlled trials (RCTs) (parallel design) comparing CFTR correctors to control in pwCF with class II mutations. Data collection and analysis: Two authors independently extracted data, assessed risk of bias and judged evidence certainty (GRADE); we contacted investigators for additional data. Main results: We included 34 RCTs (4781 participants), lasting between 1 day and 48 weeks; an extension of two lumacaftor-ivacaftor studies provided additional 96-week safety data (1029 participants). We assessed eight monotherapy RCTs (344 participants) (4PBA, CPX, lumacaftor, cavosonstat and FDL169), 16 dual-therapy RCTs (2627 participants) (lumacaftor-ivacaftor or tezacaftor-ivacaftor) and 11 triple-therapy RCTs (1804 participants) (elexacaftor-tezacaftor-ivacaftor/deutivacaftor; VX-659-tezacaftor-ivacaftor/deutivacaftor; Olacaftor (VX-440)-tezacaftor-ivacaftor; VX-152-tezacaftor-ivacaftor). Participants in 21 RCTs had the genotype F508del/F508del, in seven RCTs they had F508del/minimal function (MF), in one RCT F508del/gating genotypes, in one RCT either F508del/F508del genotypes or F508del/residual function genotypes, in one RCT either F508del/gating or F508del/residual function genotypes, and in three RCTs either F508del/F508del genotypes or F508del/MF genotypes. Risk of bias judgements varied across different comparisons. Results from 16 RCTs may not be applicable to all pwCF due to age limits (e.g. adults only) or non-standard designs (converting from monotherapy to combination therapy). Monotherapy Investigators reported no deaths or clinically relevant improvements in quality of life (QoL). There was insufficient evidence to determine effects on lung function. No placebo-controlled monotherapy RCT demonstrated differences in mild, moderate or severe adverse effects (AEs); the clinical relevance of these events is difficult to assess due to their variety and few participants (all F508del/F508del). Dual therapy In a tezacaftor-ivacaftor group there was one death (deemed unrelated to the study drug). QoL scores (respiratory domain) favoured both lumacaftor-ivacaftor and tezacaftor-ivacaftor therapy compared to placebo at all time points (moderate-certainty evidence). At six months, relative change in forced expiratory volume in one second (FEV1) % predicted improved with all dual combination therapies compared to placebo (high- to moderate-certainty evidence). More pwCF reported early transient breathlessness with lumacaftor-ivacaftor (odds ratio (OR) 2.05, 99% confidence interval (CI) 1.10 to 3.83; I2 = 0%; 2 studies, 739 participants; high-certainty evidence). Over 120 weeks (initial study period and follow-up), systolic blood pressure rose by 5.1 mmHg and diastolic blood pressure by 4.1 mmHg with twice-daily 400 mg lumacaftor-ivacaftor (80 participants). The tezacaftor-ivacaftor RCTs did not report these adverse effects. Pulmonary exacerbation rates decreased in pwCF receiving additional therapies to ivacaftor compared to placebo (all moderate-certainty evidence): lumacaftor 600 mg (hazard ratio (HR) 0.70, 95% CI 0.57 to 0.87; I2 = 0%; 2 studies, 739 participants); lumacaftor 400 mg (HR 0.61, 95% CI 0.49 to 0.76; I2 = 0%; 2 studies, 740 participants); and tezacaftor (HR 0.64, 95% CI 0.46 to 0.89; 1 study, 506 participants). Triple therapy No study reported any deaths (high-certainty evidence). All other evidence was low- to moderate-certainty. QoL respiratory domain scores probably improved with triple therapy compared to control at six months (six studies). There was probably a greater relative and absolute change in FEV1 % predicted with triple therapy (four studies each across all combinations). The absolute change in FEV1 % predicted was probably greater for F508del/MF participants taking elexacaftor-tezacaftor-ivacaftor compared to placebo (mean difference 14.30, 95% CI 12.76 to 15.84; 1 study, 403 participants; moderate-certainty evidence), with similar results for other drug combinations and genotypes. There was little or no difference in adverse events between triple therapy and control (10 studies). No study reported time to next pulmonary exacerbation, but fewer F508del/F508del participants experienced a pulmonary exacerbation with elexacaftor-tezacaftor-ivacaftor at four weeks (OR 0.17, 99% CI 0.06 to 0.45; 1 study, 175 participants) and 24 weeks (OR 0.29, 95% CI 0.14 to 0.60; 1 study, 405 participants); similar results were seen across other triple therapy and genotype combinations. Authors' conclusions: There is insufficient evidence of clinically important effects from corrector monotherapy in pwCF with F508del/F508del. Additional data in this review reduced the evidence for efficacy of dual therapy; these agents can no longer be considered as standard therapy. Their use may be appropriate in exceptional circumstances (e.g. if triple therapy is not tolerated or due to age). Both dual therapies (lumacaftor-ivacaftor, tezacaftor-ivacaftor) result in similar small improvements in QoL and respiratory function with lower pulmonary exacerbation rates. While the effect sizes for QoL and FEV1 still favour treatment, they have reduced compared to our previous findings. Lumacaftor-ivacaftor was associated with an increase in early transient shortness of breath and longer-term increases in blood pressure (not observed for tezacaftor-ivacaftor). Tezacaftor-ivacaftor has a better safety profile, although data are lacking in children under 12 years. In this population, lumacaftor-ivacaftor had an important impact on respiratory function with no apparent immediate safety concerns, but this should be balanced against the blood pressure increase and shortness of breath seen in longer-term adult data when considering lumacaftor-ivacaftor. Data from triple therapy trials demonstrate improvements in several key outcomes, including FEV1 and QoL. There is probably little or no difference in adverse events for triple therapy (elexacaftor-tezacaftor-ivacaftor/deutivacaftor; VX-659-tezacaftor-ivacaftor/deutivacaftor; Olacaftor (VX-440)-tezacaftor-ivacaftor; VX-152-tezacaftor-ivacaftor) in pwCF with one or two F508del variants aged 12 years or older (moderate-certainty evidence). Further RCTs are required in children under 12 years and those with more severe lung disease. Reference: Cochrane Database Syst Rev. 2023 Nov 20;11(11):CD010966. https://pubmed.ncbi.nlm.nih.gov/37983082/ |
| 分子式 |
C29H34FN3O4S
|
|---|---|
| 分子量 |
539.661369800568
|
| 精确质量 |
539.225
|
| 元素分析 |
C, 64.54; H, 6.35; F, 3.52; N, 7.79; O, 11.86; S, 5.94
|
| CAS号 |
1899111-41-1
|
| 相关CAS号 |
Olacaftor;1897384-89-2
|
| PubChem CID |
130203218
|
| 外观&性状 |
White to light yellow solid powder
|
| LogP |
6.3
|
| tPSA |
97
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
38
|
| 分子复杂度/Complexity |
902
|
| 定义原子立体中心数目 |
1
|
| SMILES |
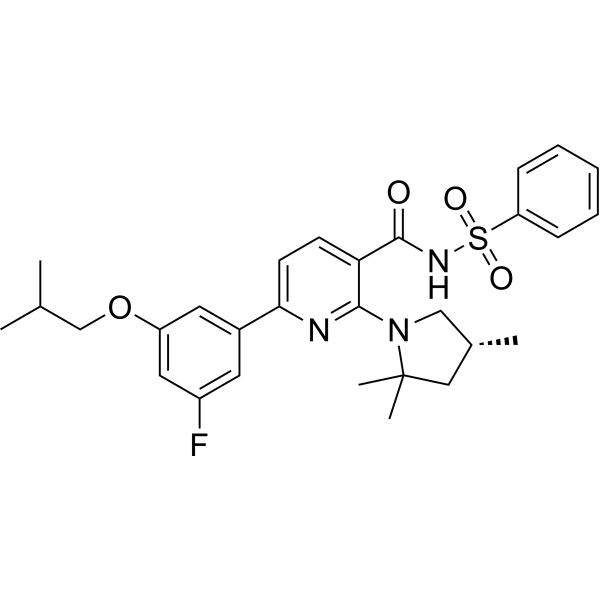
S(C1C=CC=CC=1)(NC(C1=CC=C(C2C=C(C=C(C=2)OCC(C)C)F)N=C1N1C[C@H](C)CC1(C)C)=O)(=O)=O
|
| InChi Key |
NHOUNZMCSIHKHJ-HXUWFJFHSA-N
|
| InChi Code |
InChI=1S/C29H34FN3O4S/c1-19(2)18-37-23-14-21(13-22(30)15-23)26-12-11-25(27(31-26)33-17-20(3)16-29(33,4)5)28(34)32-38(35,36)24-9-7-6-8-10-24/h6-15,19-20H,16-18H2,1-5H3,(H,32,34)/t20-/m1/s1
|
| 化学名 |
N-(benzenesulfonyl)-6-[3-fluoro-5-(2-methylpropoxy)phenyl]-2-[(4R)-2,2,4-trimethylpyrrolidin-1-yl]pyridine-3-carboxamide
|
| 别名 |
(R)-Olacaftor; 1899111-41-1; SCHEMBL19097503; (R)-VX-440;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 250 mg/mL (463.25 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (3.85 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (3.85 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8530 mL | 9.2651 mL | 18.5302 mL | |
| 5 mM | 0.3706 mL | 1.8530 mL | 3.7060 mL | |
| 10 mM | 0.1853 mL | 0.9265 mL | 1.8530 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。