| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
| 靶点 |
Mutant KRAS G12C (irreversibly binds to the switch II pocket, Ki = 11 nM for KRAS G12C-GDP; IC50 = 0.21 μM for inhibiting KRAS G12C-mediated signaling in H358 cells) [6]
|
|---|---|
| 体外研究 (In Vitro) |
- Sotorasib (AMG-510) 强效抑制KRAS G12C阳性癌细胞增殖,CellTiter-Glo实验显示IC50为0.01-0.5 μM(如H358肺腺癌细胞:0.03 μM;MIA PaCa-2胰腺癌细胞:0.12 μM)。对KRAS野生型或非G12C突变细胞无显著作用(IC50 > 10 μM)[6]
- 在H358细胞中,sotorasib(0.1-1 μM)剂量依赖性降低KRAS下游效应因子(p-ERK、p-AKT、p-S6)的磷酸化水平(Western blot),1 μM时抑制作用最强,2小时内即可检测到[6] - 该化合物(1 μM)诱导KRAS G12C阳性细胞凋亡(Annexin V/PI染色),并减少集落形成(H358细胞中减少80%)[6] |
| 体内研究 (In Vivo) |
- 在荷H358异种移植瘤(KRAS G12C)小鼠中,sotorasib(10-180 mg/kg,口服,每日1次,连续21天)引起剂量依赖性肿瘤退缩:180 mg/kg时肿瘤生长抑制率(TGI)达90%,30%完全退缩。对KRAS野生型A549异种移植瘤无显著作用[6]
- 在KRAS G12C结直肠癌患者来源异种移植(PDX)模型中,sotorasib(100 mg/kg,口服)28天后使肿瘤体积减少65%,肿瘤组织中p-ERK水平降低(免疫组化)[3] |
| 酶活实验 |
KRAS G12C结合实验:纯化的KRAS G12C-GDP蛋白与sotorasib(0.1-100 nM)共孵育,通过表面等离子体共振(SPR)分析。该化合物解离缓慢(t1/2 = 10小时),Ki为11 nM。使用发光GTP水解实验测量GTP酶活性,sotorasib(1 μM)抑制85%的KRAS G12C GTP酶活性[6]
|
| 细胞实验 |
- 增殖实验:KRAS G12C阳性细胞(H358、MIA PaCa-2)接种于96孔板,用sotorasib(0.001-10 μM)处理72小时。CellTiter-Glo检测细胞活力,非线性回归计算IC50[6]
- 信号通路Western blot:H358细胞饥饿处理后,用sotorasib(0.1-1 μM)处理2小时,裂解细胞。提取物用抗p-ERK、p-AKT和总ERK/AKT抗体检测,条带强度以β-actin标准化[6] |
| 动物实验 |
- Xenograft model: Nude mice were subcutaneously injected with H358 cells (5×10⁶). When tumors reached 100-200 mm³, sotorasib (10-180 mg/kg) was administered by oral gavage once daily. Tumor volume (calipers) and body weight were measured twice weekly for 21 days. Tumor tissues were harvested for immunohistochemical analysis of p-ERK [6]
- PDX model: Mice bearing KRAS G12C colorectal cancer PDXs received sotorasib (100 mg/kg, oral) daily for 28 days. Tumor growth was monitored, and Ki-67 (proliferation marker) expression was quantified [3] |
| 药代性质 (ADME/PK) |
- In mice, oral administration of sotorasib (10 mg/kg) showed 70% bioavailability, with peak plasma concentration (Cmax) of 2.3 μg/mL at 1 hour. It had a plasma half-life (t1/2) of 4.5 hours and good tumor penetration (tumor/plasma ratio = 3.2) [6]
- In patients, sotorasib (960 mg, oral) reached Cmax of 7.1 μg/mL at 1.5 hours, with a terminal t1/2 of 5 hours. Plasma protein binding was 95% [4] |
| 毒性/毒理 (Toxicokinetics/TK) |
- In preclinical studies, sotorasib (up to 300 mg/kg, oral) showed no significant toxicity in mice, with normal liver/kidney function markers [6]
- In clinical trials, common adverse events (≥15%) included diarrhea (34%), nausea (25%), and fatigue (21%). Grade 3/4 toxicities were rare (<5%), with no dose-limiting nephrotoxicity or hepatotoxicity [4] |
| 参考文献 | |
| 其他信息 |
- Sotorasib (AMG-510) is a first-in-class covalent inhibitor that irreversibly binds to the GDP-bound form of KRAS G12C, locking it in an inactive state [6]
- It is indicated for treating KRAS G12C-mutated non-small cell lung cancer (NSCLC) after prior systemic therapy, with FDA approval in 2021 [3][4] |
| 分子式 |
C30H30F2N6O3
|
|---|---|
| 分子量 |
560.6
|
| 精确质量 |
560.23
|
| 元素分析 |
C, 64.28; H, 5.39; F, 6.78; N, 14.99; O, 8.56
|
| 相关CAS号 |
Sotorasib;2296729-00-3;Sotorasib racemate;2252403-56-6
|
| PubChem CID |
137278711
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| LogP |
4
|
| tPSA |
102 Ų
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
41
|
| 分子复杂度/Complexity |
1030
|
| 定义原子立体中心数目 |
1
|
| SMILES |
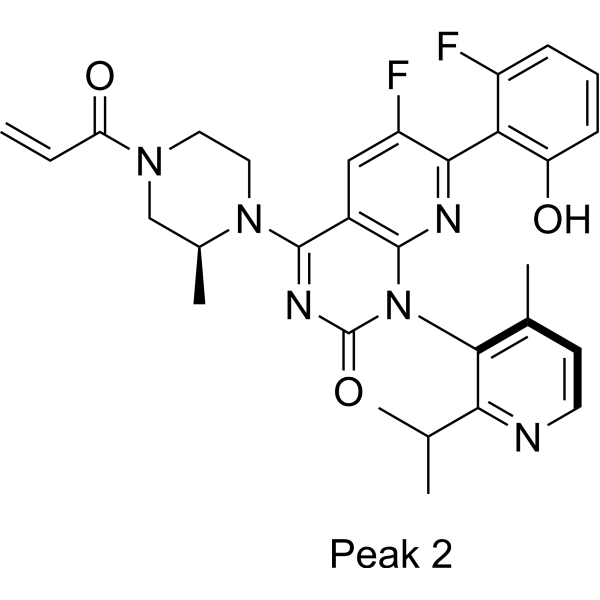
C[C@H]1CN(CCN1C2=NC(=O)N(C3=NC(=C(C=C32)F)C4=C(C=CC=C4F)O)C5=C(C=CN=C5C(C)C)C)C(=O)C=C
|
| InChi Key |
NXQKSXLFSAEQCZ-SFHVURJKSA-N
|
| InChi Code |
InChI=1S/C30H30F2N6O3/c1-6-23(40)36-12-13-37(18(5)15-36)28-19-14-21(32)26(24-20(31)8-7-9-22(24)39)34-29(19)38(30(41)35-28)27-17(4)10-11-33-25(27)16(2)3/h6-11,14,16,18,39H,1,12-13,15H2,2-5H3/t18-/m0/s1
|
| 化学名 |
6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-propan-2-ylpyridin-3-yl)-4-[(2S)-2-methyl-4-prop-2-enoylpiperazin-1-yl]pyrido[2,3-d]pyrimidin-2-one
|
| 别名 |
Sotorasib; AMG-510; 2296729-00-3; ...; Kras mutant-targeting AMG 510; ...; Sotorasib isomer;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O :< 0.1 mg/mL
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1.25 mg/mL (2.23 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 12.5 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7838 mL | 8.9190 mL | 17.8380 mL | |
| 5 mM | 0.3568 mL | 1.7838 mL | 3.5676 mL | |
| 10 mM | 0.1784 mL | 0.8919 mL | 1.7838 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。