| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
IRAK4
|
|---|---|
| 体外研究 (In Vitro) |
KT-474与CRBN和IRAK4形成三元复合物,导致IRAK4的泛素化和蛋白酶体降解。使用降解剂从myddosome中消除IRAK4有可能阻断所有下游信号传导,从而抑制TLR介导和IL-1R介导的细胞活化和细胞因子诱导。[2]
KYM-001导致IRAK4的强效E3连接酶依赖性降解。值得注意的是,与人PBMC中的IRAK4激酶抑制剂相比,KYM-001更有效地抑制TLR激活的Myddosome信号传导。在MYD88 L265P突变体ABC DLBCL系OCI-LY10中,IRAK4对>10000个其他检测到的蛋白质具有高度选择性降解。KYM-001对IRAK4的降解导致ABC DLBCL在48-72小时内的细胞周期抑制和凋亡,在MYD88突变体与MYD88-WT细胞系中具有优先活性。[3] |
| 体内研究 (In Vivo) |
口服KYM-001在人MYD88突变体ABC DLBCL的几种小鼠异种移植物模型中以耐受的剂量和时间表显示出剂量依赖性的抗肿瘤活性。在OCI-LY10模型中,肿瘤消退与IRAK4>80%的降解有关,建立了最大疗效所需的药效学效应。由于BCR信号和MYD88的改变经常在B细胞恶性肿瘤中同时发生,我们研究了IRAK4降解和BTK抑制联合活性的潜力。在具有CD79B和MYD88激活突变的OCI-LY10异种移植物模型中,用伊布替尼抑制BTK对KYM-001抗肿瘤活性具有加性作用。[3]
|
| 酶活实验 |
流动法检测PBMC中的IRAK4使用流动法评估PBMC中IRAK4的降解。冷冻的PBMC用10%FBS解冻成RPMI,90 每孔镀上μl溶液。KT-474是在10 μM起始剂量,随后进行五倍稀释和10点剂量曲线,并以0.1%的最终DMSO浓度添加。细胞和化合物在37 °C和5%二氧化碳过夜(20 h) 。孵育后,用BD Biosciences的Cytofix固定缓冲液固定细胞,并用PBS/2%FBS洗涤两次,将颗粒储存在−80 °C,直至进一步处理。在流动运行日,解冻细胞颗粒,并加入预透化染色混合物(CD3/CD14/CD56/CD19)。随后用60%甲醇将样品透化10 最小值为4 °C,然后用透化后染色混合物(CD16/IRAK4)孵育。在Attune NxT流式细胞仪上运行染色的样品。使用FlowJo分析数据,并使用GraphPad Prism使用四参数逻辑回归曲线自由拟合生成50%的抑制浓度(IC50)
IRAK4信号百分比在B细胞(CD3−/CD19+)、单核细胞(CD3−/CD19−CD14+)和淋巴细胞(通过侧散大小和CD14-确定)中确定。[2] |
| 细胞实验 |
人体细胞因子释放测定法[2]
冷冻的PBMC用热灭活的10%FBS/1%青霉素-链霉素解冻成RPMI,并于同一天以190 μl介质。KT-474、PF-06550833和DMSO对照品为每个供体制备两份。所有细胞均使用帝肯自动液体处理机给药,然后在37℃下孵育 °C和5%二氧化碳16 h、 最终测试浓度为0.0064、0.032、0.16、0.8、4、20、100和500 nM。16岁以后 用化合物预处理的h,LPS(O55:B5)(Sigma-Aldrich,L2637)或R848(Invivogen,tlrl-R848)在100 ng ml−1或10 μg ml−1最终浓度。将细胞再孵育5 h在37 °C和5%二氧化碳。测定完成后,将平板以300g离心5 最少150 小心地取出μl上清液,放入新的96孔V形底板中,并在−80下储存 °C,直至进一步分析。使用中尺度发现(MSD)人类U-plex或V-plex测定来测量细胞因子水平。在细胞因子分析当天,将上清液样品解冻,用MSD测定稀释剂稀释,并加入MSD板中。根据标准制造商的方案进一步完成测定。将细胞因子数据标准化为刺激和非刺激对照。使用MSD Discovery Workbench软件测定上清液中细胞因子的浓度。GraphPad Prism用于使用四参数逻辑回归曲线自由拟合生成IC50。[2] 人B细胞磷酸化流测定法[2] CD19+B细胞购自BioIVT或使用阴性选择试剂盒(STEMCELL Technologies,17954)在内部分离。来自n的冷冻B细胞 = 5个供体被解冻并以每孔450000个细胞的速度接种在190 μl介质在96孔U形底板中。KT-474、PF-06550833和DMSO对照品为每个供体制备两份。所有细胞均使用帝肯自动液体处理机给药,然后在37℃下孵育 °C和5%二氧化碳16 h、 最终测试浓度为0.12、0.489、1.95、7.81、31.2、125、500和2000 nM。16岁以后 化合物预处理的h,CpG-B(InvivoGen,tlrl-2006)以2.5添加 μM最终浓度60 培养后,将等体积的BD Cytofix固定缓冲液加入孔中。细胞用PBS+2%FBS洗涤,然后在冰上透化30 分钟,使用冷BD Perm缓冲液III(BD Biosciences,558050)。在用荧光标记的抗体(藻红蛋白(PE)磷酸-p65,克隆K10-895.12.50(BD Biosciences,558423))染色30分钟之前,再次洗涤细胞 然后在使用Attune NxT流式细胞仪采集之前洗涤细胞两次,每孔10000次。使用FlowJo分析数据,并使用GraphPad Prism使用四参数逻辑回归曲线自由拟合生成IC50。 |
| 动物实验 |
Tumor xenograft studies were conducted by implanting human ABC DLBCL lines into immunocompromised mouse strains and assessing tumor volume.[3]
IRAK4 in human PBMC, ABC DLBCL cell lines and xenografts was quantified by immunoassays or targeted MS/MS. Myddosome signaling was monitored by mRNA and phosphoprotein endpoints. Cell viability and cell cycle were monitored by flow cytometry. Tumor xenograft studies were conducted by implanting human ABC DLBCL lines into immunocompromised mouse strains and assessing tumor volume.[3] |
| 参考文献 | |
| 其他信息 |
Toll-like receptor-driven and interleukin-1 (IL-1) receptor-driven inflammation mediated by IL-1 receptor-associated kinase 4 (IRAK4) is involved in the pathophysiology of hidradenitis suppurativa (HS) and atopic dermatitis (AD). KT-474 (SAR444656), an IRAK4 degrader, was studied in a randomized, double-blind, placebo-controlled phase 1 trial where the primary objective was safety and tolerability. Secondary objectives included pharmacokinetics, pharmacodynamics and clinical activity in patients with moderate to severe HS and in patients with moderate to severe AD. KT-474 was administered as a single dose and then daily for 14 d in 105 healthy volunteers (HVs), followed by dosing for 28 d in an open-label cohort of 21 patients. Degradation of IRAK4 was observed in HV blood, with mean reductions after a single dose of ≥93% at 600-1,600 mg and after 14 daily doses of ≥95% at 50-200 mg. In patients, similar IRAK4 degradation was achieved in blood, and IRAK4 was normalized in skin lesions where it was overexpressed relative to HVs. Reduction of disease-relevant inflammatory biomarkers was demonstrated in the blood and skin of patients with HS and patients with AD and was associated with improvement in skin lesions and symptoms. There were no drug-related infections. These results, from what, to our knowledge, is the first published clinical trial using a heterobifunctional degrader, provide initial proof of concept for KT-474 in HS and AD to be further confirmed in larger trials. ClinicalTrials.gov identifier: NCT04772885 .[1]
Purpose: This work assessed the antitumor activity of selective small molecule IRAK4 degraders in human ABC DLBCL cell lines in vitro and in tumor xenograft models in vivo, alone and in combination with BTK inhibition.[2] Introduction: ABC DLBCL comprises approximately 45% of DLBCL and has a worse outcome with R-CHOP chemotherapy compared to GCB DLBCL. Activating mutations in MYD88 occur in 30-40% of ABC DLBCL; L265P, the most prevalent MYD88 mutation, causes constitutive assembly and activation of the Myddosome. IRAK4 kinase and scaffolding functions are essential for full signaling through the Myddosome to NFκB and MAPK pathways. Kymera Therapeutics is using a chemical knockdown strategy to develop heterobifunctional small molecule IRAK4 degraders, exemplified by KYM-001, for the treatment of MYD88-driven lymphomas. [2] Methods: IRAK4 in human PBMC, ABC DLBCL cell lines and xenografts was quantified by immunoassays or targeted MS/MS. Myddosome signaling was monitored by mRNA and phosphoprotein endpoints. Cell viability and cell cycle were monitored by flow cytometry. Tumor xenograft studies were conducted by implanting human ABC DLBCL lines into immunocompromised mouse strains and assessing tumor volume.[2] Key data: KYM-001 led to potent E3 ligase-dependent degradation of IRAK4. Notably, KYM-001 more effectively inhibited TLR-activated Myddosome signaling compared to IRAK4 kinase inhibitors in human PBMC. Degradation was highly selective for IRAK4 vs >10,000 other detected proteins in the MYD88 L265P mutant ABC DLBCL line OCI-LY10. IRAK4 degradation by KYM-001 resulted in cell cycle inhibition and apoptosis within 48-72 h in ABC DLBCL, with preferential activity in MYD88-mutant vs MYD88-WT cell lines. Oral dosing of KYM-001 showed dose-dependent antitumor activity in several mouse xenograft models of human MYD88-mutant ABC DLBCL at tolerated doses and schedules. In the OCI-LY10 model, tumor regression was associated with >80% degradation of IRAK4, establishing the pharmacodynamic effect required for maximal efficacy. Since alterations in BCR signaling and MYD88 frequently co-occur in B-cell malignancies, we investigated the potential for combined activity of IRAK4 degradation and BTK inhibition. In the OCI-LY10 xenograft model, which has activating mutations in both CD79B and MYD88, BTK inhibition with ibrutinib had an additive effect on KYM-001 antitumor activity.[2] Conclusions: KYM-001 is a first-in-class, potent, selective and orally active IRAK4 degrader that causes tumor regression in ABC-DLBCL models. Degradation of IRAK4 removes both the kinase and scaffolding functions of IRAK4, and may be superior to kinase inhibition alone. These data support IRAK4 degraders as a promising new therapeutic opportunity for MYD88-driven lymphoma, both alone and in combination with other targeted approaches such as BTK inhibition.[2] |
| 分子式 |
C44H49F2N11O6
|
|---|---|
| 分子量 |
865.926775693893
|
| 精确质量 |
865.383
|
| 元素分析 |
C, 61.03; H, 5.70; F, 4.39; N, 17.79; O, 11.09
|
| CAS号 |
2432994-31-3
|
| PubChem CID |
146599824
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
2.5
|
| tPSA |
172Ų
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
13
|
| 可旋转键数目(RBC) |
11
|
| 重原子数目 |
63
|
| 分子复杂度/Complexity |
1790
|
| 定义原子立体中心数目 |
2
|
| SMILES |
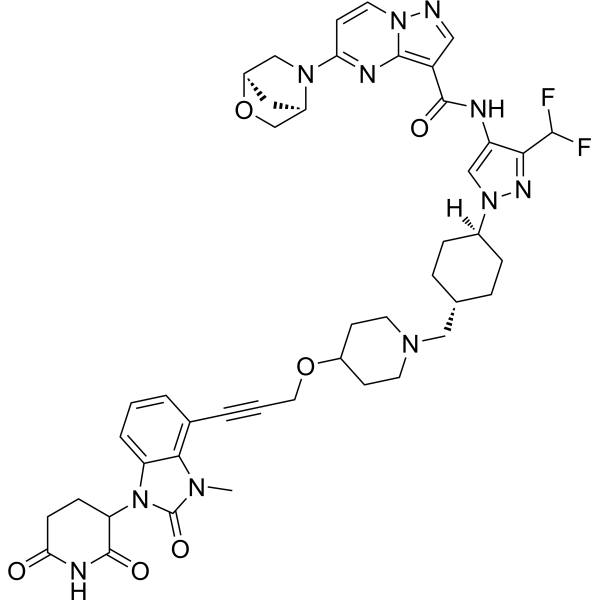
CN1C2=C(C=CC=C2N(C1=O)C3CCC(=O)NC3=O)C#CCOC4CCN(CC4)CC5CCC(CC5)N6C=C(C(=N6)C(F)F)NC(=O)C7=C8N=C(C=CN8N=C7)N9C[C@H]1C[C@@H]9CO1
|
| InChi Key |
NQGKNAVUMAHSQN-PKIOHZLWSA-N
|
| InChi Code |
InChI=1S/C44H49F2N11O6/c1-52-39-27(4-2-6-34(39)57(44(52)61)35-11-12-37(58)50-43(35)60)5-3-19-62-30-13-16-53(17-14-30)22-26-7-9-28(10-8-26)56-24-33(38(51-56)40(45)46)48-42(59)32-21-47-55-18-15-36(49-41(32)55)54-23-31-20-29(54)25-63-31/h2,4,6,15,18,21,24,26,28-31,35,40H,7-14,16-17,19-20,22-23,25H2,1H3,(H,48,59)(H,50,58,60)/t26?,28?,29-,31-,35?/m1/s1
|
| 化学名 |
5-((1R,4R)-2-oxa-5-azabicyclo[2.2.1]heptan-5-yl)-N-(3-(difluoromethyl)-1-(4-((4-((3-(1-(2,6-dioxopiperidin-3-yl)-3-methyl-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-4-yl)prop-2-yn-1-yl)oxy)piperidin-1-yl)methyl)cyclohexyl)-1H-pyrazol-4-yl)pyrazolo[1,5-a]pyrimidine-3-carboxamide
|
| 别名 |
KT-474; KYM-001; KT474; PROTAC IRAK4 degrader-7; KT-474; KT474; N-[3-(Difluoromethyl)-1-[trans-4-[[4-[[3-[1-(2,6-dioxo-3-piperidinyl)-2,3-dihydro-3-methyl-2-oxo-1H-benzimidazol-4-yl]-2-propyn-1-yl]oxy]-1-piperidinyl]methyl]cyclohexyl]-1H-pyrazol-4-yl]-5-(1R,4R)-2-oxa-5-azabicyclo[2.2.1]hept-5-ylpyrazolo[1,5-a]pyrimidine-3-carboxamide; N-[3-(Difluoromethyl)-1-[4-[[4-[3-[1-(2,6-dioxopiperidin-3-yl)-3-methyl-2-oxobenzimidazol-4-yl]prop-2-ynoxy]piperidin-1-yl]methyl]cyclohexyl]pyrazol-4-yl]-5-[(1R,4R)-2-oxa-5-azabicyclo[2.2.1]heptan-5-yl]pyrazolo[1,5-a]pyrimidine-3-carboxamide; 2SXR65P7E3; SCHEMBL21998241; KYM001
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~115.48 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (2.89 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (2.89 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.1548 mL | 5.7741 mL | 11.5483 mL | |
| 5 mM | 0.2310 mL | 1.1548 mL | 2.3097 mL | |
| 10 mM | 0.1155 mL | 0.5774 mL | 1.1548 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。