| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g | |||
| Other Sizes |

| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
ITS ONSET OF ACTION BY ORAL ROUTE IS ABOUT 320 MIN...ITS DURATION OF ACTION BY THE ORAL ROUTE IS USUALLY SOMEWHAT LONGER AND ABSORPTION IS LESS ERRATIC THAN NEOSTIGMINE, WHICH ARE ADVANTAGES. Plasma concn of pyridostigmine was determined in 2 nursing mothers who were receiving oral doses of pyridostigmine bromide, 120-300 mg daily. The drug was not detectable in infant plasma and there were no signs of drug effects in the infant. Metabolism / Metabolites PYRIDOSTIGMINE AND ITS QUATERNARY ALCOHOL ARE...THE PREDOMINANT ENTITIES FOUND IN URINE AFTER ADMIN OF THIS DRUG TO MAN. Biological Half-Life After admin of pyridostigmine bromide (200 nmol/kg, iv) to human subjects, the disposition half-life was 0.6-1.78 min and terminal half-life was 14.81-37.01 min. Clearance was 9.3-26.5 ml/min/kg which was greater than the presumptive value for glomerular filtration rate and the vol of distribution was 246.5-833.9 ml/kg. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Because of the low levels of pyridostigmine in breastmilk, amounts ingested by the infant are small and infant serum levels are very low. Pyridostigmine is not expected to cause any adverse effects in breastfed infants. Most mothers with myasthenia gravis are able to nurse successfully with pyridostigmine treatment, but occasionally breastfeeding must be discontinued to avoid excessive fatigue in the mother. ◉ Effects in Breastfed Infants A woman was taking pyridostigmine 120 mg every 4 to 5 hours for myasthenia gravis. Her breastfed (extent not stated) infant reportedly thrived and had no cholinergic side effects. Two infants whose mothers were taking pyridostigmine 3 and 5 mg/kg daily during pregnancy and lactation were exclusively breastfed. Both infants gained weight and developed normally and had no signs of cholinergic side effects. ◉ Effects on Lactation and Breastmilk Relevant published information in nursing mothers was not found as of the revision date. In animals, cholinergic drugs increase oxytocin release, and have variable effects on serum prolactin. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. In a case series of 69 pregnancies in 65 women with myasthenia gravis over 27 years, 49 patients received pyridostigmine during pregnancy and lactation. Lactation data were available for 33 patients, and 25 of them nursed successfully, although the number of these mothers who were taking pyridostigmine was not given. Nursing was sometimes disrupted to avoid exhaustion in the mother. Interactions ACTIONS OF ANTICHOLINESTERASE AGENTS ON AUTONOMIC EFFECTOR CELLS & ON CORTICAL & SUBCORTICAL SITES IN CNS, WHERE RECEPTORS ARE LARGELY OF MUSCARINIC TYPE, ARE BLOCKED BY ATROPINE. /ANTI-CHE AGENTS/ A SINGLE CASE HAS BEEN REPORTED IN WHICH METHOCARBAMOL MAY HAVE IMPAIRED THE THERAPEUTIC EFFECT OF PYRIDOSTIGMINE BROMIDE IN A PATIENT WITH MYASTHENIA GRAVIS. In anesthetized patients, pyridostigmine bromide mestinon 10 mg iv antagonized the neuromuscular block induced by d-tubocurarine chloride 0.29 mg/kg/hr. Adequate recovery from the neuromuscular block should be judged by the return of the twitch height to the control level & also by the restoration of well-sustained tetanus 30 cycles/sec to the level seen before d-tubocurarine chloride administration. Prolonged effect of succinylcholine after neostigmine and pyridostigmine administration in patients with renal failure. The amino acid gamma-aminobutyric acid (GABA), when given as supplement to pyridostigmine bromide reduced the incidence of malformations in chicken embryos. |
| 参考文献 |
Neuroscience.2013 Aug 29;246:391-6;Luminescence.2011 Nov-Dec;26(6):510-7.
|
| 其他信息 |
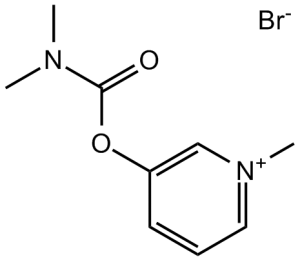
Pyridostigmine bromide is a pyridinium salt.
Pyridostigmine is a drug that has been approved by the U.S. Food and Drug Administration (FDA) under the brand name Mestinon to treat a condition called myasthenia gravis and under the brand name Regonol to reverse the effects of muscle relaxants. In addition, pyridostigmine has been approved for use by military personnel as a pretreatment for exposure to the chemical nerve agent Soman. It is also being studied as an investigational drug to treat HIV infection. As an investigational HIV drug, pyridostigmine belongs to a group of drugs called immune modulators. Immune modulators (also called immunomodulators) are substances that help to activate, boost, or restore normal immune function. Researchers are currently studying whether pyridostigmine added to antiretroviral therapy (ART) can help a person with HIV increase their CD4 count. Pyridostigmine Bromide is the bromide salt form of pyridostigmine, a quaternary ammonium carbamate derivative and a acetylcholinesterase inhibitor. Pyridostigmine bromide binds reversibly to acetylcholinesterase active sites in the peripheral nervous system, thereby preventing the breakdown of acetylcholine. This leads to an accumulation of acetylcholine at cholinergic synapses and facilitates transmission of impulses across the neuromuscular junction. Mediated through muscarinic receptors, this agent increases contraction of bronchial and intestinal smooth muscle and the exocrine glands secretions, while it causes paralysis of the skeletal muscles mediated through nicotinic receptors. In addition, pyridostigmine is used as a reversible blocking agent to prevent organophosphates from binding to the acetylcholinesterase receptors and thereby protect the nervous system from the effects of nerve agents such as Soman. A cholinesterase inhibitor with a slightly longer duration of action than NEOSTIGMINE. It is used in the treatment of myasthenia gravis and to reverse the actions of muscle relaxants. See also: Pyridostigmine (has active moiety). Mechanism of Action ...PHARMACOLOGICAL EFFECTS OF ANTICHOLINESTERASE AGENTS ARE DUE PRIMARILY TO PREVENTION OF HYDROLYSIS OF /ACH/ ACETYLCHOLINE BY ACHE /ACETYLCHOLINESTERASE/ @ SITES OF CHOLINERGIC TRANSMISSION. TRANSMITTER THUS ACCUMULATES, AND THE ACTION OF ACH /ACETYLCHOLINE/ THAT IS LIBERATED BY CHOLINERGIC IMPULSES OR THAT LEAKS FROM THE NERVE ENDING IS ENHANCED. Following admin of pyridostigmine bromide to rats, erythrocyte acetylcholinesterase activity recovered only slowly due to the covalent nature of inhibition. The logarithm of the plasma concn of pyridostigmine bromide was linearly related to the increase in tibialis twitch tension due to facilitation of neuromuscular transmission. Of 12 analogs of pyridostigmine prepared by reacting 2-substituted 3-pyridinols with the desired carbamoyl chloride 2-iodo-3-(dimethylcarbamoyloxy)pyridine methiodide was the most active inhibitor of acetylcholinesterase and butyrylcholinesterase. The progressive inhibition curves for AChE and BuChE are compared and related to ionic attraction and steric requirements of the inhibitors. Therapeutic Uses Cholinesterase Inhibitors; Parasympathomimetics A QUATERNARY AMMONIUM ANTICHOLINESTERASE DRUG ...PRINCIPAL USE IS IN THE TREATMENT OF MYASTHENIA GRAVIS. ... DOSAGE: THE EQUIVALENT PARENTERAL DOSE OF...PYRIDOSTIGMINE IS APPROX 1/30TH OF THE ORAL DOSE. PYRIDOSTIGMINE HAS A SLOWER ONSET (13 MIN) THAN EDROPHONIUM (3 MIN) OR NEOSTIGMINE (6-8 MIN), BUT A LONGER DURATION OF ACTION THAN EITHER. FOR THIS REASON, IT HAS BEEN RECOMMENDED FOR PATIENTS WITH RENAL IMPAIRMENT. For more Therapeutic Uses (Complete) data for PYRIDOSTIGMINE BROMIDE (9 total), please visit the HSDB record page. Drug Warnings BROMIDE SENSITIVITY OCCASIONALLY OCCURS. Maternal Medication usually Compatible with Breast-Feeding: Pyridostigmine: Reported Sign or Symptom in Infant or Effect on Lactation: None. /from Table 6/ |
| 分子式 |
C9H13BRN2O2
|
|
|---|---|---|
| 分子量 |
261.12
|
|
| 精确质量 |
260.016
|
|
| CAS号 |
101-26-8
|
|
| 相关CAS号 |
Pyridostigmine-d6 bromide;2375858-08-3;Pyridostigmine-d3 bromide
|
|
| PubChem CID |
7550
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
0.9613 g/cm3 (20ºC)
|
|
| 沸点 |
88 (25 torr)
|
|
| 熔点 |
154 °C
|
|
| 折射率 |
1.48 (20ºC)
|
|
| tPSA |
33.42
|
|
| 氢键供体(HBD)数目 |
0
|
|
| 氢键受体(HBA)数目 |
3
|
|
| 可旋转键数目(RBC) |
2
|
|
| 重原子数目 |
14
|
|
| 分子复杂度/Complexity |
183
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
VNYBTNPBYXSMOO-UHFFFAOYSA-M
|
|
| InChi Code |
InChI=1S/C9H13N2O2.BrH/c1-10(2)9(12)13-8-5-4-6-11(3)7-8;/h4-7H,1-3H3;1H/q+1;/p-1
|
|
| 化学名 |
(1-methylpyridin-1-ium-3-yl) N,N-dimethylcarbamate;bromide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (9.57 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (9.57 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (9.57 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 100 mg/mL (382.97 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.8297 mL | 19.1483 mL | 38.2966 mL | |
| 5 mM | 0.7659 mL | 3.8297 mL | 7.6593 mL | |
| 10 mM | 0.3830 mL | 1.9148 mL | 3.8297 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05334485 | Not yet recruiting | Drug: Pyridostigmine Bromide Other: Placebo |
Postoperative Ileus | Stefan Holubar MD MS FACS, FASCRS | December 2024 | Phase 2 Phase 3 |
| NCT05110417 | Terminated | Drug: Pyridostigmine Bromide | Pompe Disease | Eastern Virginia Medical School | May 20, 2021 | Phase 4 |
| NCT05110417 | Recruiting | Drug: Pyridostigmine Bromide 60 Milligrams (mg) |
Dysphonia, Spastic Dysphonia Laryngeal Dystonia |
Eastern Virginia Medical School | May 20, 2021 | Phase 4 |
| NCT05603715 | Recruiting | Drug: Pyridostigmine Bromide | Parkinson Disease Constipation |
University of Vermont Medical Center | August 10, 2022 | Phase 2 |
| NCT02941328 | Completed | Drug: Pyridostigmine Drug: Placebo |
Spinal Muscular Atrophy SMA Kugelberg-Welander Disease |
UMC Utrecht | December 2015 | Phase 2 |