| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Non-depolarizing neuromuscular blocker; muscle relaxant
Neuromuscular nicotinic acetylcholine receptor (nAChR) [2][3][4] |
|---|---|
| 体外研究 (In Vitro) |
罗库溴铵以浓度依赖性方式降低了所有预处理隔膜(P <.01,n = 6)和正常隔膜(50% 抑制浓度 [IC(50)],9.84 [9.64-10.04] μM,间接引起的抽搐张力,平均值 [95% 置信区间])[1]。罗库溴铵对儿童和成人的 ED95 几乎相同。其在儿童中的半衰期比成人短,其作用与维库溴铵类似。使用标准剂量的胆碱酯酶抑制药物很容易逆转罗库溴铵[2]。设置最大阻滞、将抽搐高度从 25% 恢复到 75% 以及将抽搐高度从 25% 恢复到 75% 的时间分别为 1.7 (32)、53 (19) 和 20 (37) 分钟。 3]。
在离体大鼠膈神经-膈肌标本中,Rocuronium Bromide(ORG 9426 Bromide,罗库溴铵溴化物)(0.1–10 μM)以剂量依赖性方式抑制乙酰胆碱(ACh)诱导的肌肉收缩,产生非去极化型神经肌肉阻滞。1 μM浓度下,收缩幅度降低70%,起效时间为2.5分钟,作用持续时间(50%恢复时间)为15分钟[2] - 该化合物对毒蕈碱型乙酰胆碱受体的内在活性极低,浓度高达10 μM时,对离体豚鼠回肠中ACh诱导的平滑肌收缩无显著抑制作用[2] |
| 体内研究 (In Vivo) |
仅 8.7±5.7% (SD) 和 6.0±2.8% 的 ORG 9426 和 ORG 9616 给药剂量分别通过尿液消除。另一方面,未结扎肾蒂的猫将 ORG 9616 的 35.7±12.2% 和 46.8±9.7% 排泄到胆汁中,以及 ORG 9426 给药剂量的 54.4±9.2% 和 52.4±9.2% 分别排泄到胆汁中 [4]。
在笑气、氟烷和芬太尼麻醉的患者中,静脉注射Rocuronium Bromide(0.6 mg/kg)可产生95%的神经肌肉阻滞,起效时间为60秒。临床持续时间(25%恢复时间)为31分钟,总恢复时间(25%至95%恢复)为17分钟[3] - 在戊巴比妥麻醉的猫中,Rocuronium Bromide(0.5 mg/kg,静脉注射)诱导90%的神经肌肉阻滞,起效时间为45秒。阻滞持续时间(50%恢复时间)为28分钟,给药后42分钟实现完全恢复(抽搐高度95%)[4] - 在对氧磷诱导的有机磷中毒大鼠模型中,Rocuronium Bromide(0.3 mg/kg,静脉注射)诱导的神经肌肉阻滞持续时间较对照组延长2.3倍。对氧磷给药后5分钟注射碘解磷定(20 mg/kg,静脉注射)可逆转该延长效应,使阻滞持续时间降至对照组的1.2倍[1] - 人体中增加Rocuronium Bromide剂量(1.0 mg/kg,静脉注射)可将临床持续时间延长至48分钟,且未显著增加不良反应[3] |
| 酶活实验 |
酶联免疫吸附试验(ELISA)[5]
为了检测用RB或不用RB处理12小时的成纤维细胞分泌的细胞因子,使用CXCL12、IL8、HGF和TGF-β的抗体进行ELISA方法。 制备富含神经肌肉nAChR的兔骨骼肌细胞膜悬液。将系列稀释的Rocuronium Bromide(0.01–100 μM)与细胞膜悬液、[3H]-α-银环蛇毒素(选择性nAChR配体)在测定缓冲液中混合,25°C孵育90分钟。玻璃纤维滤膜过滤去除未结合配体,液体闪烁计数器检测结合配体的放射性强度,计算特异性结合抑制率[2] |
| 细胞实验 |
分别在含有10%胎牛血清和抗生素的DMEM和1640中培养人EC TE-1和ECA-109细胞,以及人成纤维细胞HS-27、TIG-1和CRL-7815细胞。成纤维细胞在多种条件下预处理,然后传代并使用μ-玻片2孔系统与EC细胞共培养。药物细胞的处理条件如下:RB(罗库溴铵)以10–320μg/mL处理12h;用10μg/mL处理12小时的人CXCL12重组蛋白(RP-8658);雷帕霉素(500 nM,8小时)[5]。
|
| 动物实验 |
0.3 mg/kg and 0.6 mg/kg
Cats The neuromuscular blocking effects and pharmacokinetics of ORG 9426, 1.5 mg/kg and ORG 9616, 1.2 mg/kg iv, two new nondepolarizing neuromuscular blocking drugs, were studied in 28 cats (i.e., 14 cats with each drug) with and without renal pedicle ligation. A gas chromatographic assay was used to determine the concentrations of ORG 9426 and ORG 9616 and its desacetyl metabolites in plasma, urine, bile, and liver. The duration of neuromuscular blockade of both drugs was not altered by ligation of renal pedicles. Plasma clearance of ORG 9426 was slower in cats with ligated renal pedicles (P less than 0.01). With ORG 9616, mean elimination half-life was slower and mean residence time longer in cats with renal pedicle ligation. Otherwise, there was no significant differences with any pharmacokinetic variables in cats with and without renal pedicle ligation. Only 8.7 +/- 5.7% (SD) and 6.0 +/- 2.8% of an injected dose of ORG 9426 and ORG 9616 was excreted into the urine, respectively. Conversely, 54.4 +/- 9.2% and 52.4 +/- 9.2% of an injected dose of ORG 9426 and 35.7 +/- 12.2% and 46.8 +/- 9.7% of ORG 9616 were excreted into the bile in cats without and with renal pedicle ligation, respectively. Finally, 21.3 +/- 6.5% and 33.5 +/- 15.6% of ORG 9426 and 14.0 +/- 3.2% and 18.1 +/- 5.6% of ORG 9616 were in the liver 6 h after injection in cats without and with renal pedicle ligation respectively. The authors were able to account for the biodisposition of 84.4% and 85.9% of an injected dose of ORG 9426 in cats without and with renal pedicle ligation respectively.[4] Xenograft mouse study[5] Four-week-old female BALB/c nude mice were used for xenograft tumor experiments. The transplantation and drug administration were carried out in strict accordance with the International Committee's Guide for the Care and Use of Laboratory Animals. For xenograft tumor experiment, 5 × 106 TE-1 or ECA-109 cells mixed with matrix-gel were subcutaneously inoculated into the back of the right upper limb of nude mice (3 mice per group). For systematical administration, after 1 week subcutaneous inoculation, RB with different concentrations (0, 10 and 20 mg/kg) [23] were administrated with the nude mice every 2 days for additional 2 weeks. For local administration, the gradient concentrations of RB (0, 40 and 80 μg per mouse) were given in the connective tissue layer underneath the tumor every two days for additional 2 weeks. Then mice were executed with carbon dioxide asphyxia, xenograft tumors were measured and removed for histochemical analysis. Immunohistochemistry (IHC) was used to detect the expression change of ATG5 and CXCL12 influenced by RB treatment in vivo, xenograft tumors and their adjacent tissues were fixed, embedded within paraffin, and sectioned routinely. Antibodies against ATG5 at 1:500 dilution and CXCL12 at 1:1000 dilution were used respectively. Cat neuromuscular block model: Adult cats (2.5–4 kg) were anesthetized with pentobarbital sodium (30 mg/kg, i.v.) and mechanically ventilated. The sciatic nerve was stimulated electrically (0.1 Hz, 0.2 ms duration), and twitch tension of the tibialis anterior muscle was recorded. Rocuronium Bromide (0.25, 0.5, 1.0 mg/kg, i.v.) was administered, and onset time, duration of block (50% and 95% recovery), and recovery profile were measured [4] - Rat organophosphate poisoning model: Male SD rats (250–300 g) were anesthetized with urethane (1.5 g/kg, i.p.). Paraoxon (0.5 mg/kg, i.v.) was administered to induce poisoning, followed by Rocuronium Bromide (0.3 mg/kg, i.v.) to induce neuromuscular block. In the treatment group, pralidoxime (20 mg/kg, i.v.) was injected 5 minutes after paraoxon administration. Twitch tension of the gastrocnemius muscle was recorded to evaluate block duration and reversal [1] |
| 药代性质 (ADME/PK) |
In humans, after intravenous administration of Rocuronium Bromide (0.6 mg/kg), the volume of distribution at steady state (Vdss) was 0.28 L/kg, and the plasma clearance was 3.7 mL/min/kg. The elimination half-life (t1/2β) was 73 minutes [3]
- In cats, intravenous Rocuronium Bromide (0.5 mg/kg) had a Vdss of 0.32 L/kg, clearance of 4.2 mL/min/kg, and elimination half-life of 68 minutes [4] - The drug is primarily metabolized in the liver via deacetylation, and approximately 70% of the dose is excreted in bile, with 10–15% excreted in urine as unchanged drug [2][3] - Rocuronium Bromide has a plasma protein binding rate of 25–30% in humans [3] |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Limited information on the use of rocuronium during breastfeeding indicates that no adverse infant effects occur. Because it is short acting, highly polar and poorly absorbed orally, it is not likely to reach the breastmilk in high concentration or to reach the bloodstream of the infant. When a combination of anesthetic agents is used for a procedure, follow the recommendations for the most problematic medication used during the procedure. General anesthesia for cesarean section using rocuronium as a component may delay the onset of lactation. ◉ Effects in Breastfed Infants Four mothers who were breastfeeding 3- to 5-month-old infants underwent general anesthesia were given intravenous propofol and remifentanil as induction agents and rocuronium 0.5 mg/kg for intubation. After induction, propofol was stopped and xenon inhalation was used to maintain anesthesia for between 57 and 70 minutes. Infants resumed breastfeeding from 1.5 to 5 hours after the end of surgery. None of the infants had noticeable symptoms of dizziness or drowsiness. All infants fared well at home after their mothers were discharged with no adverse events noticed at home. ◉ Effects on Lactation and Breastmilk A randomized study compared the effects of cesarean section using general anesthesia, spinal anesthesia, or epidural anesthesia, to normal vaginal delivery on serum prolactin and oxytocin as well as time to initiation of lactation. General anesthesia was performed using propofol 2 mg/kg and rocuronium 0.6 mg/kg for induction, followed by sevoflurane and rocuronium 0.15 mg/kg as needed. After delivery, patients in all groups received an infusion of oxytocin 30 international units in 1 L of saline, and 0.2 mg of methylergonovine if they were not hypertensive. Fentanyl 1 to 1.5 mcg/kg was administered after delivery to the general anesthesia group. Patients in the general anesthesia group (n = 21) had higher post-procedure prolactin levels and a longer mean time to lactation initiation (25 hours) than in the other groups (10.8 to 11.8 hours). Postpartum oxytocin levels in the nonmedicated vaginal delivery group were higher than in the general and spinal anesthesia groups. Common clinical adverse effects include mild histamine release (reported in 5–8% of patients), manifested as flushing and hypotension; these effects are dose-dependent and transient [2][3] - No significant hepatotoxicity or nephrotoxicity was observed in clinical trials or animal studies, with no consistent changes in serum liver enzymes or renal function parameters [2][3][4] - The intravenous LD50 of Rocuronium Bromide in rats is >8 mg/kg, indicating low acute toxicity [2] - Concurrent administration with organophosphate compounds (e.g., paraoxon) prolongs neuromuscular block duration, which can be reversed by pralidoxime [1] - No significant drug-drug interactions were observed with common anesthetics (nitrous oxide, halothane, fentanyl) [3] |
| 参考文献 | |
| 其他信息 |
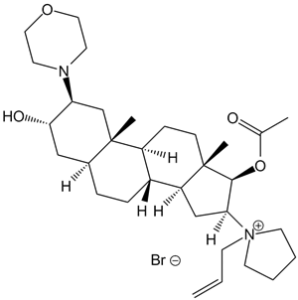
Rocuronium bromide is the organic bromide salt of a 5alpha androstane compound having 3alpha-hydroxy-, 17beta-acetoxy-, 2beta-morpholino- and 16beta-N-allyllyrrolidinium substituents. It has a role as a neuromuscular agent and a muscle relaxant. It is an organic bromide salt and a quaternary ammonium salt. It contains a rocuronium. It derives from a hydride of a 5alpha-androstane.
Rocuronium Bromide is the bromide salt form of rocuronium, an intermediate-acting quaternary aminosteroid with muscle relaxant property. Rocuronium bromide competitively binds to the nicotinic receptor at the motor end plate, and antagonizes acetylcholine binding, which results in skeletal muscle relaxation and paralysis. An androstanol non-depolarizing neuromuscular blocking agent. It has a mono-quaternary structure and is a weaker nicotinic antagonist than PANCURONIUM. See also: Rocuronium (has active moiety). Rocuronium Bromide (ORG 9426 Bromide) is a non-depolarizing neuromuscular blocking agent (NMBA) used clinically to induce muscle relaxation during general anesthesia [2][3][4] - Its mechanism of action involves competitive antagonism of neuromuscular nicotinic acetylcholine receptors, preventing ACh from binding and activating the receptor, thereby inhibiting muscle contraction [2][4] - It is characterized by rapid onset (60–90 seconds at clinical doses) and intermediate duration of action, making it suitable for short to moderate-duration surgical procedures [3][4] - The drug is administered intravenously, with clinical doses ranging from 0.6 mg/kg (induction of block) to 0.15 mg/kg (maintenance doses) [3] - It is reversed by acetylcholinesterase inhibitors (e.g., neostigmine) combined with antimuscarinic agents (e.g., atropine) [2] |
| 分子式 |
C32H53N2O4.BR
|
|
|---|---|---|
| 分子量 |
609.68
|
|
| 精确质量 |
608.318
|
|
| 元素分析 |
C, 63.04; H, 8.76; Br, 13.11; N, 4.59; O, 10.50
|
|
| CAS号 |
119302-91-9
|
|
| 相关CAS号 |
143558-00-3; 119302-91-9 (bromide)
|
|
| PubChem CID |
441351
|
|
| 外观&性状 |
Typically exists as white to off-white solids at room temperature
|
|
| 密度 |
162-164°C
|
|
| 熔点 |
162-1640C
|
|
| LogP |
1.308
|
|
| tPSA |
59
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
6
|
|
| 重原子数目 |
39
|
|
| 分子复杂度/Complexity |
898
|
|
| 定义原子立体中心数目 |
10
|
|
| SMILES |
[Br-].O(C(C([H])([H])[H])=O)[C@@]1([H])[C@]([H])(C([H])([H])[C@@]2([H])[C@]3([H])C([H])([H])C([H])([H])[C@@]4([H])C([H])([H])[C@@]([H])([C@]([H])(C([H])([H])[C@]4(C([H])([H])[H])[C@@]3([H])C([H])([H])C([H])([H])[C@@]21C([H])([H])[H])N1C([H])([H])C([H])([H])OC([H])([H])C1([H])[H])O[H])[N+]1(C([H])([H])C([H])=C([H])[H])C([H])([H])C([H])([H])C([H])([H])C1([H])[H]
|
|
| InChi Key |
OYTJKRAYGYRUJK-UHFFFAOYSA-M
|
|
| InChi Code |
InChI=1S/C32H53N2O4.BrH/c1-5-14-34(15-6-7-16-34)28-20-26-24-9-8-23-19-29(36)27(33-12-17-37-18-13-33)21-32(23,4)25(24)10-11-31(26,3)30(28)38-22(2)35;/h5,23-30,36H,1,6-21H2,2-4H3;1H/q+1;/p-1
|
|
| 化学名 |
[(2S,3S,5S,8R,9S,10S,13S,14S,16S,17R)-3-hydroxy-10,13-dimethyl-2-morpholin-4-yl-16-(1-prop-2-enylpyrrolidin-1-ium-1-yl)-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl] acetate;bromide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (3.41 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 配方 2 中的溶解度: 100 mg/mL (164.02 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6402 mL | 8.2010 mL | 16.4020 mL | |
| 5 mM | 0.3280 mL | 1.6402 mL | 3.2804 mL | |
| 10 mM | 0.1640 mL | 0.8201 mL | 1.6402 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04512313 | Completed | Drug: Rocuronium 0,3mg/kg Drug: Rocuronium 0,9mg/kg |
Neuromuscular Blockade Neuromuscular Blockade, Residual Anesthesia Intubation Complication |
Rigshospitalet, Denmark | December 17, 2020 | Not Applicable |
| NCT02376595 | Completed | Drug: Rocuronium Bromide | Neuromuscular Blockade | Pontificia Universidad Catolica de Chile | March 2013 | |
| NCT05476952 | Completed | Procedure: he patients who are administered rocuronium according to LBW were categorized as "LBW", and The group number for those administer according to TBW was categorized as "K". |
Body Weight Changes Rocuronium Intubation Conditions |
DUYGU DEMİROZ | January 30, 2023 | |
| NCT02827435 | Recruiting | Drug: Rocuronium bromide | End Stage Renal Disease Transplantation |
Seoul National University Hospital | July 1, 2016 | Not Applicable |
| NCT00124735 | Completed Has Results | Drug: Rocuronium bolus maintenance Drug: rocuronium continuous infusion maintenance |
Anesthesia | Merck Sharp & Dohme LLC | October 2004 | Phase 3 |
|
|---|
|
|