| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| Other Sizes |
|
| 靶点 |
β-Lactamases
|
|---|---|
| 体外研究 (In Vitro) |
Xeruborbactam disodium 是鲍曼不动杆菌 D 类碳青霉烯酶的有效抑制剂 [1]。铜绿假单胞菌的主要 MDR 外排泵的活性对 Xeruborbactam 二钠没有影响,这是相对于上一代硼酸 β-内酰胺酶抑制剂 (BLI) Vaborbactam 的重大进步 [1]。 Xeruborbactam 二钠是一种含有多种 β-内酰胺抗生素的抗生素,由于其广谱 β-内酰胺酶抑制特性、多种 β-内酰胺抗生素的活性增强以及对流出和渗透性的不同敏感性,当与酰胺抗生素联合使用时,这些抗生素是有用的抑制剂内在的抵抗机制[1]。
QPX7728(化合物35)显示出非常广泛的抑制谱,包括B类和D类酶,并且几乎不受孔蛋白修饰和外排的影响[2] 生化评价[2] QPX7728与丝氨酸β-内酰胺酶表现出“慢结合”动力学,而与金属酶则表现出“快速开启-快速关闭”动力学。表11显示了使用硝基头孢(NCF)或亚胺培南(IMI)作为底物从过表达重组大肠杆菌菌株中纯化的各种β-内酰胺酶的抑制常数(Ki)。除IMP-1外,所有酶的Ki值均<100 nM。化合物35在抗鲍曼不动杆菌D类酶OXA-48和OXA-23以及抗B类金属酶NDM-1、VIM-1和IMP-1方面明显优于对照品瓦泊巴坦和阿维巴坦。 微生物学[2] QPX7728/化合物35(固定浓度为8μg/mL)与头孢吡肟、头孢噻嗪和美罗培南联合使用,对包括肺炎克雷伯菌(n=511)、大肠杆菌(n=297)、阴沟肠杆菌复合物(n=88)和其他生物体(n=119)在内的肠杆菌群进行了评估;其中507株表达ESBL酶,508株为碳青霉烯类耐药株。化合物35还对碳青霉烯类耐药鲍曼不动杆菌(n=503)和铜绿假单胞菌(n=500)进行了测试。MIC50/MIC90值如表12所示。还显示了与市售药物头孢他啶-阿维巴坦和美罗培南-瓦泊巴坦的比较数据。这三种组合共有35种,对产生ESBLs的肠杆菌以及表达KPC和OXA-48碳青霉烯酶的菌株都非常有效。在表达金属酶(NDM和VIM)的菌株中,头孢吡肟和美罗培南的效力分别提高到1和2μg/mL的MIC90值,而头孢噻嗪几乎没有获益。这归因于MBL的高头孢噻嗪水解活性,以及对MBL的抑制作用较低(与丝氨酸酶相比)35。对碳青霉烯类耐药鲍曼不动杆菌,只有美罗培南的效力得到了充分增强,而对铜绿假单胞菌,所有三种组合的MIC90均≤8μg/mL。对外排泵和孔蛋白修饰过表达和/或不足的菌株的研究表明,35株在很大程度上不受这些常见耐药机制的影响(数据未显示)。有趣的是,35本身显示出较弱的抗菌活性,对肠杆菌的MIC为16μg/mL(范围<1至≥32μg/mL);一般来说,这种活性的浓度远高于强化MIC,因此不太可能是组合活性的主要因素。总体而言,这些研究的结果证明了35的广泛用途,并且临床医生可以使用多种组合。 |
| 体内研究 (In Vivo) |
感染模型的疗效[2]
在针对肺炎克雷伯氏菌KP1244菌株的24小时中性粒细胞减少小鼠大腿感染模型中评估化合物35(QPX7728)(图6)。该菌株表达KPC-3、SHV-11和SHV-12,对碳青霉烯类抗生素具有抗性,对美罗培南的MIC>64μg/mL;在8μg/mL的35浓度下,美罗培南的MIC为0.25μg/mL。美罗培南和QPX7728的剂量均为每2小时给药一次,持续24小时(每天12剂指定剂量)。在这项研究中,每2小时给予300 mg/kg剂量的美罗培南无法控制感染,而与35的0.5-1 mg/kg联合给药可产生静态效果,10 mg/kg可使CFU/大腿减少>1个对数(相对于治疗开始)。 |
| 酶活实验 |
β-内酰胺酶抑制Ki值的测定[2]
使用硝基头孢(NCF)或亚胺培南作为报告底物,通过分光光度法测定从过表达重组大肠杆菌菌株中纯化的β-内酰胺酶的Ki抑制值。将酶与不同浓度的抑制剂在反应缓冲液中混合(50 mM磷酸钠,pH 7.0,0.1 mg/mL BSA,金属酶加20μM ZnCl2),并在37°C下孵育10分钟。加入50μM NCF,在SpectraMax板读数器上每10秒记录490 nm的底物裂解曲线10分钟。在SpectraMax平板读数仪上每30秒记录294nm的底物裂解曲线,持续30分钟,用于亚胺培南。Ki值采用Waley方法计算。 QPX7728对丝氨酸蛋白酶的影响[2] 所有酶和底物均来自商业来源,并根据制造商的协议进行了一些修改后的测试。简而言之,将50μL稀释的酶与50μL不同浓度的抑制剂和50μL相应的缓冲液混合(表15)。反应混合物在37℃下孵育10分钟。随后,加入50μL相应的底物,在SpectraMax M2平板读数器上监测吸光度或荧光30分钟。4-(2-氨基乙基)苯磺酰氟盐酸盐(AEBSF)和亮肽用作阳性对照。计算并展示了相对于“未治疗”对照的反应速率。IC50值基于产生50%酶抑制的抑制剂浓度计算。 含有多种孔蛋白和外排突变组合的工程细菌菌株。[1] 构建肺炎克雷伯菌、铜绿假单胞菌和鲍曼不动杆菌菌株的外排/孔蛋白同源图谱,以评估各种分子决定因素对QPX7728全细胞抗生素增强活性的影响。 早些时候描述了一组具有孔蛋白(ompK35和ompK36)和外排(acrAB-tolC)突变的肺炎克雷伯菌同源KPC-3产生菌株(其中KPC-3携带在天然质粒pKpQIL上)的构建(22)。通过将质粒pUCP24-KPC-2转化为各种突变体,构建了过表达或缺乏MDR RND外排泵MexAB-OprM、MexCD-OprJ、MexEF-OprN和MexXY-OprM并产生或缺乏碳青霉烯孔蛋白OprD的铜绿假单胞菌产KPC-2菌株的同源组。通过将携带来自临床分离株AB1177的OXA-23的天然质粒偶联到各种外排突变体中,构建了过表达MDR-RND外排泵AdeABC和AdeIJK的鲍曼不动杆菌产OXA-23同源菌株。 |
| 细胞实验 |
抗菌药物敏感性试验。[1]
根据临床和实验室标准研究所(CLSI)的方法,使用内部制备的面板对细菌分离株进行肉汤微量稀释敏感性试验。使用符合《临床微生物学程序手册》中Moody程序的棋盘式分析来评估不同浓度的QPX7728或瓦泊巴坦对各种抗生素MIC的影响。PV50和最大增强值(PVmax)用于确定β-内酰胺酶抑制剂的效力。PV50被定义为达到50%抗生素增效作用所需的最低BLI浓度,或将抗生素MIC降低到产β-内酰胺酶菌株MIC和相应缺乏β-内酰酶菌株MIC之间MIC范围的中点所需的BLI浓度。MIC中点是产生β-内酰胺酶的菌株和缺乏β-内酰酶的菌株的抗生素MIC值的几何平均值,计算为产生β-外酰胺酶和缺乏β内酰胺酶菌株的抗生素MIC值乘积的平方根。 PVmax被定义为最大增强值,即将抗生素MIC降低到缺乏β-内酰胺酶(KPC)的亲本菌株所需的BLI浓度(对应于KPC的完全抑制)。 单体形成的测定[2] 称取试验化合物,放入1.5mL Eppendorf管中。基于活性组分,以1.00、10.0或100mg/mL的化合物浓度制备水溶液。减去20μL的体积,留出添加酸或碱的空间来调节pH值。测量溶液pH值。然后根据需要通过添加酸或碱来调节pH值,直到读数在7.6-8范围内。在确保所有化合物都在溶液中后进行最终pH测量。在样品制备后不到半小时内,将溶液以0.1-5μL的体积注入LC-UV。在Excel ACE 5 Super C18 2.1 mm×100 mm柱上,使用0.1%TFA水溶液作为流动相A,0.085%TFA甲醇溶液作为流动相中B,以0.6 mL/min的流速进行洗脱,梯度为9分钟,在90%B下保持3.9分钟,运行时间为18分钟。在带宽为4nm的220、254和300nm处测量吸收。 敏感性测试[2] 如CLSI文件M07-A11(2018)所述,使用临床和实验室标准研究所(CLSI)肉汤微量稀释法测定MIC。 |
| 动物实验 |
Pharmacokinetic Studies in Rats [2]
After acclimation, rats (n = 3/dose level) were administered either single intravenous infusions (in 0.9% saline) of QPX7728 at 30, 100, or 300 mg/kg or at 30, 100, 300, or 1000 mg/kg via the oral route (in water). Intravenous doses were infused over 0.5 h via an indwelling femoral vein cannula, while oral doses were administered via a bead tipped oral gavage. Plasma (∼0.3 mL) samples were collected from each rat at designated time points up to 24 h. Blood samples were centrifuged within 5 min of collection at 12 000g for 5 min to obtain plasma. The plasma samples were analyzed using an HPLC–MS method. PK analysis was performed using WinNonlin. Mouse Efficacy Studies [2] Swiss-Webster mice were rendered neutropenic by the administration of cyclophosphamide and were infected under isoflurane anesthesia by intramuscular injection of K. pneumoniae KP1244 (inoculum 1 × 106) in both thighs (meropenem MIC of >64 μg/mL; meropenem MIC in the presence of 8 μg/mL QPX7728 was 0.25 μg/mL). Treatments (formulated in water) were administered every 2 h by the intraperitoneal route, starting 2 h postinfection. Animals were sacrificed 24 h after the start of treatment, and the thighs were removed, homogenized, and plated to determine bacterial counts. |
| 药代性质 (ADME/PK) |
Pharmacokinetics [1]
The pharmacokinetics of QPX7728 by intravenous (iv) and oral (po) administration were evaluated in the rat at multiple doses (Table 14). The systemic exposure by iv administration, as shown by the Cmax and AUC values, increased in a dose proportional manner. Overall the parameters are similar to those of most β-lactam antibiotics, evidencing high Cmax and AUC, short half-life, and low volume of distribution. Plasma protein binding in the rat is 85%. QPX7728/Compound 35 also displays oral bioavailability (F) in fasted rats, with values ranging between 43% and 53% at doses of 30–100 mg/kg, whereas it declined to 24–28% at higher doses. Given its polarity (log D7.4 = −2.85), we speculate that active transport may be involved in oral uptake, which may be saturated at higher doses. The compound was well tolerated at all doses. |
| 毒性/毒理 (Toxicokinetics/TK) |
Safety [2]
Compound 35/QPX7728 was studied in a 7-day pilot toxicology study at daily doses of 30, 100, and 300 mg/kg in rats (five males, five females per dose level) administered by iv infusion. No changes were observed (tissue histology and clinical chemistry). |
| 参考文献 |
[1]. The Impact of Intrinsic Resistance Mechanisms on Potency of QPX7728, a New Ultra-Broad-Spectrum Beta-lactamase Inhibitor of Serine and Metallo Beta-Lactamases in Enterobacteriaceae, Pseudomonas aeruginosa, and Acinetobacter baumannii.Antimicrob Agents Chemother. 2020 May 21;64(6):e00552-20.
[2]. Discovery of Cyclic Boronic Acid QPX7728, an Ultrabroad-Spectrum Inhibitor of Serine and Metallo-β-lactamases. J Med Chem. 2020;63(14):7491-7507. |
| 其他信息 |
Despite major advances in the β-lactamase inhibitor field, certain enzymes remain refractory to inhibition by agents recently introduced. Most important among these are the class B (metallo) enzyme NDM-1 of Enterobacteriaceae and the class D (OXA) enzymes of Acinetobacter baumannii. Continuing the boronic acid program that led to vaborbactam, efforts were directed toward expanding the spectrum to allow treatment of a wider range of organisms. Through key structural modifications of a bicyclic lead, stepwise gains in spectrum of inhibition were achieved, ultimately resulting in QPX7728 (35). This compound displays a remarkably broad spectrum of inhibition, including class B and class D enzymes, and is little affected by porin modifications and efflux. Compound 35 is a promising agent for use in combination with a β-lactam antibiotic for the treatment of a wide range of multidrug resistant Gram-negative bacterial infections, by both intravenous and oral administration.[1]
QPX7728 is an ultrabroad-spectrum boronic acid beta-lactamase inhibitor that demonstrates inhibition of key serine and metallo-beta-lactamases at a nanomolar concentration range in biochemical assays with purified enzymes. The broad-spectrum inhibitory activity of QPX7728 observed in biochemical experiments translates into enhancement of the potency of many beta-lactams against strains of target pathogens producing beta-lactamases. The impacts of bacterial efflux and permeability on inhibitory potency were determined using isogenic panels of KPC-3-producing isogenic strains of Klebsiella pneumoniae and Pseudomonas aeruginosa and OXA-23-producing strains of Acinetobacter baumannii with various combinations of efflux and porin mutations. QPX7728 was minimally affected by multidrug resistance efflux pumps either in Enterobacteriaceae or in nonfermenters, such as P. aeruginosa or A. baumannii Against P. aeruginosa, the potency of QPX7728 was further enhanced when the outer membrane was permeabilized. The potency of QPX7728 against P. aeruginosa was not affected by inactivation of the carbapenem porin OprD. While changes in OmpK36 (but not OmpK35) reduced the potency of QPX7728 (8- to 16-fold), QPX7728 (4 μg/ml) nevertheless completely reversed the KPC-mediated meropenem resistance in strains with porin mutations, consistent with the lesser effect of these mutations on the potency of QPX7728 compared to that of other agents. The ultrabroad-spectrum beta-lactamase inhibition profile, combined with enhancement of the activity of multiple beta-lactam antibiotics with various sensitivities to the intrinsic resistance mechanisms of efflux and permeability, indicates that QPX7728 is a useful inhibitor for use with multiple beta-lactam antibiotics.[1] QPX7728 is a new boronate BLI with potent inhibitory activity against both serine and metallo-beta-lactamases. The broad-spectrum inhibitory activity of QPX7728 previously observed in cell-free biochemical experiments using purified enzymes translates into enhancement of the activity of many beta-lactams against strains of target pathogens producing beta-lactamases.[1] The potent inhibitory activity of QPX7728 in whole cells is driven in part by a lack of efflux by major transporters from Gram-negative bacteria at concentrations that are relevant for beta-lactamase inhibition. A lack of efflux of QPX7728 is particularly important for inhibitory activity in P. aeruginosa and represents a significant improvement over the earlier boronate BLI vaborbactam. Mutations in outer membrane porin proteins of Enterobacteriaceae are associated with the reduced potency of many antibiotics and beta-lactamase inhibitors. The potency of QPX7728 in Enterobacteriaceae is affected by the inactivation of the major general porins OmpK35/OmpF and OmpK36/OmpC much less than the boronate inhibitor vaborbactam is.[1] The potent, ultrabroad-spectrum inhibitory activity of QPX7728 shown with multiple beta-lactam antibiotics with various sensitivities to beta-lactamases as well as intrinsic resistance mechanisms makes it an ideal candidate for multiple product development strategies. Conventional approaches for product configurations include the development of a fixed-combination beta-lactam–beta-lactamase inhibitor for which there is a well-established regulatory path. An important limitation of this strategy is identifying a partner beta-lactam that, in combination with the BLI, has the best overall activity against most but perhaps not all target pathogens with different mixtures of resistance mechanisms. Another approach would be the development of QPX7728 as a stand-alone drug product that could be coadministered with different existing beta-lactam antibiotics, depending on the mechanisms present in the specific pathogen. This approach has several clinical and regulatory implications but could be an important step toward individualized treatment of infections caused by drug-resistant pathogens by taking into account local epidemiology, patient factors, and antibiotic stewardship. The multiple benefits of this strategy should encourage the establishment of a defined path for future regulatory approval.[1] |
| 分子式 |
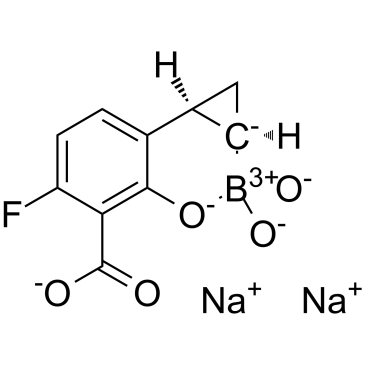
C??H?BFNA?O?
|
|---|---|
| 分子量 |
281.94
|
| 精确质量 |
284.024
|
| CAS号 |
2170848-99-2
|
| 相关CAS号 |
Xeruborbactam;2170834-63-4
|
| PubChem CID |
162642679
|
| 外观&性状 |
Off-white to yellow solid powder
|
| tPSA |
89.8
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
19
|
| 分子复杂度/Complexity |
372
|
| 定义原子立体中心数目 |
2
|
| SMILES |
[B-]1([C@@H]2C[C@@H]2C3=C(O1)C(=C(C=C3)F)C(=O)[O-])(O)O.[Na+].[Na+]
|
| InChi Key |
RLVWXGRGTAHSCI-BNTLRKBRSA-M
|
| InChi Code |
InChI=1S/C10H9BFO5.2Na/c12-7-2-1-4-5-3-6(5)11(15,16)17-9(4)8(7)10(13)14;;/h1-2,5-6,15-16H,3H2,(H,13,14);;/q-1;2*+1/p-1/t5-,6-;;/m1../s1
|
| 化学名 |
disodium;(2S,4R)-9-fluoro-5,5-dihydroxy-6-oxa-5-boranuidatricyclo[5.4.0.02,4]undeca-1(7),8,10-triene-8-carboxylate
|
| 别名 |
QPX 7728OH disodium; QPX-7728 OH disodium; QPX7728-OH disodium; QPX7728-OH (disodium); 2170848-99-2; PD165287;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~352.16 mM)
H2O : ~76.67 mg/mL (~270.00 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 66.67 mg/mL (234.79 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.5469 mL | 17.7343 mL | 35.4685 mL | |
| 5 mM | 0.7094 mL | 3.5469 mL | 7.0937 mL | |
| 10 mM | 0.3547 mL | 1.7734 mL | 3.5469 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。