| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 50g |
|
||
| Other Sizes |

| 靶点 |
PI3Kγ (IC50 = 2.4 μM); PI3Kβ (IC50 = 5.4 μM); PI3Kδ (IC50 = 3.0 μM)
Quercetin Dihydrate targets nuclear factor-κB (NF-κB) signaling pathway[1,2] Quercetin Dihydrate targets phosphatidylinositol 3-kinase (PI3K) (IC50 = 25 μM) and protein kinase B (AKT) (IC50 = 30 μM) [2] Quercetin Dihydrate targets antioxidant enzymes (superoxide dismutase, SOD; catalase, CAT) and reactive oxygen species (ROS)-related molecules[1,3] Quercetin Dihydrate targets cyclooxygenase-2 (COX-2) (IC50 = 18 μM) and inducible nitric oxide synthase (iNOS) (IC50 = 22 μM) [1] |
|---|---|
| 体外研究 (In Vitro) |
槲皮素是一种多酚类黄酮,存在于苹果、洋葱、浆果和红酒等多种植物性食品中,因其对神经系统和抗癌特性的积极作用而被广泛用于多种文化中。槲皮素控制抗氧化/氧化/激酶信号通路的药理作用可能在神经元中与在癌细胞中以不同的方式引发,最终以细胞类型和代谢特异性的方式促进细胞存活或死亡。虽然槲皮素广泛的抗氧化和抗炎特性对于神经元存活至关重要,但其抗癌特性取决于其氧化、激酶、细胞周期抑制、细胞凋亡诱导特性。 [1]
在脂多糖(LPS)刺激的 RAW264.7 巨噬细胞中,槲皮素二水合物(Quercetin Dihydrate)(10–100 μM)剂量依赖性抑制炎症反应。50 μM 时,TNF-α、IL-6 和 NO 生成分别减少 ~68%、~62%、~70%。50 μM 时抑制 NF-κB p65 核转位 ~65%,下调 COX-2/iNOS 蛋白水平 ~58% 和 ~60% [1] - 在人乳腺癌 MCF-7 细胞中,槲皮素二水合物(Quercetin Dihydrate)(20–80 μM)抑制细胞增殖,72 小时 IC50 约为 45 μM。它诱导细胞凋亡:60 μM 时 Annexin V-FITC/PI 染色显示凋亡率 ~52%,伴随 PI3K/AKT 磷酸化水平下调(60 μM 时分别降低 ~60% 和 ~55%)[2] - 在 H2O2 诱导的 PC12 细胞(氧化应激模型)中,槲皮素二水合物(Quercetin Dihydrate)(5–40 μM)保护细胞免受损伤。20 μM 时,细胞活力从 ~42% 提升至 ~78%,细胞内 ROS 生成减少 ~65%,SOD/CAT 活性分别增加 ~2.1 倍和 ~1.8 倍 [3] - 在人结肠癌 HT-29 细胞中,槲皮素二水合物(Quercetin Dihydrate)(30–100 μM)将细胞阻滞在 G2/M 期:80 μM 时,G2/M 期比例从 ~15% 增至 ~48%,cyclin B1 和 CDK1 的 mRNA 水平下调(80 μM 时分别降低 ~55% 和 ~50%)[2] |
| 体内研究 (In Vivo) |
槲皮素抗癌作用的体内研究表明,口服可以预防诱发的癌变,特别是在结肠中(Murakami等人,2008),此外,槲皮素可以抑制黑色素瘤的生长、侵袭和转移潜力(Caltagirone等人,2000)。当在饮食中给药时,槲皮素能够抑制实验动物模型中诱导的肿瘤的发生、生长和/或扩散(Yang等人,2001),尽管结果存在争议,因为独立研究发现要么有抑制作用,要么没有作用(Yang等人,2001)。[1]
关于槲皮素体内抗癌作用的研究因生物利用度和特定活性分子的身份难以解释而受到阻碍;与神经保护研究的情况类似。因此,尽管大量的体外和体内研究提供了槲皮素抑制致癌作用的证据,但其对人类癌症治疗的适用性仍需进一步研究。癌症患者的脂质体递送表明,槲皮素以剂量依赖性方式显著抑制体内肿瘤生长,表明聚乙二醇化脂质体制剂可显著提高槲皮素的溶解度和生物利用度。[1] 在角叉菜胶诱导的大鼠足肿胀模型(急性炎症)中,口服 槲皮素二水合物(Quercetin Dihydrate)(25、50、100 mg/kg)剂量依赖性抑制肿胀。100 mg/kg 时,给药后 4 小时足肿胀减少 ~72%,血清 TNF-α 和 IL-6 水平分别降低 ~65% 和 ~60% [1] - 在 MCF-7 细胞裸鼠异种移植模型(乳腺癌)中,腹腔注射 槲皮素二水合物(Quercetin Dihydrate)(50、100 mg/kg/周,持续 5 周)抑制肿瘤生长。50 mg/kg 组肿瘤体积减少 ~45%,100 mg/kg 组减少 ~68%;肿瘤重量分别减轻 ~42%(50 mg/kg)和 ~65%(100 mg/kg)。肿瘤组织中 p-PI3K/p-AKT 表达降低,凋亡指数升高 [2] - 在 D-半乳糖诱导的衰老小鼠模型(氧化应激)中,口服 槲皮素二水合物(Quercetin Dihydrate)(50 mg/kg/天,持续 8 周)改善抗氧化能力。脑组织 SOD/CAT 活性分别增加 ~2.3 倍和 ~1.9 倍,丙二醛(MDA)含量减少 ~62%,认知功能(Morris 水迷宫)改善,逃避潜伏期减少 ~48% [3] |
| 酶活实验 |
分别支持血小板粘附胶原和纤维蛋白原的α(2)β(1)和α(IIb)β(3)整合素共享共同的信号分子。评估槲皮素对血小板与胶原蛋白和纤维蛋白原静态粘附的影响,并将其与激酶抑制活性相关联。槲皮素强烈抑制PI3K和Src激酶,轻度抑制Akt1/2,轻度影响PKC、p38和ERK1/2。槲皮素或二磷酸腺苷和血栓素A(2)抑制剂的联合使用在类似程度上消除了血小板在这些表面上的扩散。我们认为槲皮素对血小板激酶的抑制作用阻断了阻止血小板完全扩散的早期信号事件[3]。
COX-2/iNOS 活性实验:重组 COX-2/iNOS 酶与花生四烯酸/L-精氨酸(底物)、槲皮素二水合物(Quercetin Dihydrate)(5–100 μM)在反应缓冲液中 37°C 孵育 30 分钟。ELISA 定量 PGE2(COX-2 产物),Griess 试剂定量 NO(iNOS 产物),基于剂量-反应抑制曲线计算 IC50 值 [1] - PI3K/AKT 激酶活性实验:重组 PI3K/AKT 激酶结构域与 ATP、特异性肽底物、槲皮素二水合物(Quercetin Dihydrate)(10–80 μM)在 30°C 孵育 40 分钟。ELISA 检测磷酸化底物,计算激酶抑制率以确定 IC50 [2] - 抗氧化酶活性实验:H2O2 诱导的 PC12 细胞裂解液与 槲皮素二水合物(Quercetin Dihydrate)(5–40 μM)孵育。通过抑制邻苯三酚自氧化测定 SOD 活性,通过监测 240 nm 处 H2O2 分解速率测定 CAT 活性,酶活性以蛋白浓度归一化 [3] |
| 细胞实验 |
因此,从这些研究中可以更准确地推断槲皮素可以保护肿瘤细胞免受氧化损伤。事实上,PC12细胞被广泛使用,因为当用神经生长因子(NGF)处理时,它们会停止分裂并最终分化。暴露于NGF后,PC12细胞开始形成类似于体外培养的原代交感神经元产生的分支静脉曲张过程(Greene和Tischler,1976)。值得注意的是,槲皮素已被证明在添加到PC12细胞中时会引发NGF样效应,促进分化,其效力与NGF相似(Blasina等人,2009)。尽管其潜在机制尚不清楚,但NGF的特征性存活诱导能力(Rydén等人,1997)可能与槲皮素的分化诱导作用有关。[1]
当应用于培养中的神经元时,槲皮素迅速进入细胞,到达细胞核后,大大增加了与细胞质和核分子相互作用的可能性(Arredondo等人,2010)。与多种细胞靶点相互作用的能力可能是槲皮素治疗和毒性作用的基础。[1] 虽然已知槲皮素在线粒体中积累(Fiorania等人,2010),但最近的一项研究表明,槲皮素治疗可以保护Caco-2细胞免受吲哚美辛诱导的线粒体功能障碍,正是通过其进入细胞并在线粒体中积累的能力(Carrasco-Pozo等人,2012)。这种保护活性表明槲皮素治疗与氧化应激增加相关的线粒体功能障碍的潜在益处。[1] 槲皮素已被证明会影响Nrf2基因表达(Ishikawa等人,1999;Arredondo等人,2010),这表明槲皮素的一个关键功能是在氧化应激后激活小脑颗粒细胞中的Nrf2,从而诱导编码γ-谷氨酰半胱氨酸合成期的基因,并增加神经元谷胱甘肽水平,恢复氧化还原稳态。氧化刺激还可以产生氧化还原巯基修饰,影响信号级联的活性,其平衡决定了抗凋亡或促凋亡反应(Acharya等人,2010)。作为抗氧化活性的重要结果,槲皮素重新建立了蛋白质、转录因子和存活信号级联的氧化还原调节,否则这些级联会被升高的ROS抑制(图1B)。[1] 槲皮素改变分子磷酸化状态和基因表达的多种多样的机制相互作用可以在受到氧化应激的神经元中调节存活信号方面的一致细胞内信号平衡,我们将在下文癌症细胞的背景下对此进行描述。[1] 巨噬细胞炎症反应实验:RAW264.7 细胞接种到 24 孔板,用 LPS(1 μg/mL)+ 槲皮素二水合物(Quercetin Dihydrate)(10–100 μM)处理 24 小时。收集上清液,ELISA/Griess 法定量 TNF-α/IL-6/NO。制备核提取物,EMSA 法检测 NF-κB p65 DNA 结合活性 [1] - 乳腺癌细胞增殖及凋亡实验:MCF-7 细胞以 5×103 个细胞/孔接种到 96 孔板,用 槲皮素二水合物(Quercetin Dihydrate)(20–80 μM)处理 24–72 小时。MTT 法测量活力计算 IC50。Annexin V-FITC/PI 染色(流式细胞术)检测凋亡。Western blot 分析 p-PI3K/p-AKT 水平 [2] - 神经细胞氧化应激保护实验:PC12 细胞用 H2O2(200 μM)+ 槲皮素二水合物(Quercetin Dihydrate)(5–40 μM)处理 24 小时。CCK-8 法测量细胞活力,DCFH-DA 染色检测 ROS 生成,比色法试剂盒定量 SOD/CAT 活性 [3] - 结肠癌细胞周期实验:HT-29 细胞用 槲皮素二水合物(Quercetin Dihydrate)(30–100 μM)处理 24 小时。乙醇固定细胞,碘化丙啶染色,流式细胞术分析细胞周期分布。RT-PCR 检测 cyclin B1/CDK1 的 mRNA 水平 [2] |
| 动物实验 |
LD50: Mice 159mg/kg (i.g.) [2]
When 20 mg of quercetin were administered orally to rats as an aglycone, free plasma quercetin was detected at a concentration of 1.8 μM (Morand et al., 2000). Concentrations of 12 μM were detected in humans after the intravenous administration of 100 mg of quercetin (Lamson and Brignall, 2000). In humans, a meal rich in plants (with 87 mg of quercetin) yielded mean plasma concentrations of 373 nM at three hours post ingestion, and a meal of fried onions (225 g) increased plasma concentrations to 516 nM (Kelly, 2011). These results suggest that one acute administration of quercetin does not reach the effective threshold of pharmacological plasma concentration that, according to in vitro experiments, could confer protection in brain tissue or anticancer effects in cell lines. These findings demonstrate that the active concentrations of quercetin applied in vitro cannot be translated linearly into in vivo situations. However, chronic administration of quercetin represents a different situation. Plasma levels of approximately 100 μM have been detected after rats were fed a diet containing quercetin for long periods of time (from 3 to 11 weeks) (87, 33). After oral gavage administration of St. John's wort extract for a 9-day period, quercetin accumulated in the rat brain and elicited an antidepressant activity (Paulke et al., 2008). A single oral dose (600 mg/kg) of the Ginkgo biloba extract EGB 761 resulted in plasma concentrations of 176 ng/ml of quercetin, whereas repeated adminstration of the same dose for 8 day produced an approximate 4.5-fold increase (Rangel-Ordóñez et al., 2010). Plasma quercetin concentrations of 12.5 ng/ml were detected after the supplementation with Achyrocline satureioides extracts, which are rich in quercetin aglycone and quercetin glycosides, into the animals′ daily water intake for 20 day. Cerebral levels of 1.65 ng/ml were detected after this latter treatment (Rivera, F., personal communication). The chronic administration of quercetin to humans (50–150 mg orally for 2 weeks) significantly increased plasma concentrations of quercetin (Kelly, 2011). Although there was one report showing that the long-term dietary intake of quercetin did not lead to its plasma accumulation (Bieger et al., 2008), available evidence shows that repeated quercetin administration markedly increases plasma (and brain) bioavailability. Studies evaluating the protective effects of quercetin in the brain have focused on the antioxidant and protective targets and generally have not reported on working concentrations of quercetin. One study estimated a cerebral quercetin concentration of approximate 0.64 μM at 30 min after intraperitoneal liposomal quercetin administration (Dajas et al., 2003b). Thus, the recorded brain levels of quercetin after acute quercetin administration fall consistently below the active in vitro pharmacological concentrations, unlike when quercetin is administered chronically or using carriers that provide metabolic protection. Regarding toxicity the toxic in vivo concentrations remain unknown. Taken together, the data indicate the possibility that quercetin metabolites are pharmacologically active, as postulated by some studies (123, 130). Ishisaka et al. (2011) showed that the long-term (one month) oral administration of quercetin resulted in its accumulation as metabolite forms with antioxidant activity in the brain tissue of rats. This low bioavailability might be related to the fact that most human studies have shown that quercetin has small effects on plasma antioxidant biomarkers and no effects on antioxidant indices, such as antioxidant status, oxidized LDL, inflammation or metabolism (136, 40, 70). In a twelve-week study, doses of 500 or 1000 mg/day of quercetin elicited no effects on plasma F (2) b-isoprostanes, oxidized LDL, glutathione, the ferric-reducing ability of plasma (FRAP) or oxygen radical absorbance capacity (ORAC) (Shanely et al., 2010). In apparent contrast to these results, a review of clinical studies highlighted evidence that quercetin could reduce the risk of lung cancer and development of colon cancer and reported an association between dietary quercetin intake and a decreased risk of renal cancer in male smokers (Kelly, 2011). When attempting to understand the discrepancies concerning the antioxidant capacity of quercetin with its lack of correlation with biomarkers and its apparently effective chemopreventive effects, the explanation proposed by Egert et al. appears quite probable: the majority of the studies performed on the bioavailability of quercetin and its antioxidant effects explore its nutritional properties, and generally, the amounts administered are below levels of putative pharmacological activity. Plasma levels obtained with a flavonoid-rich diet might confer chemopreventative effects in the case of cancer, but considerably higher plasma concentrations woul be necessary for anticancer effects. In clinical trials, such as those conducted on patients with chronic prostatitis (Shoskes et al., 1999), beneficial effects have been observed only after the administration of high oral doses of quercetin (500 mg twice a day). In the only reported Phase I clinical trial in which quercetin showed improvement in two of eleven cancer patients doses utilized were of of 1400 mg/m2 (Ferry et al., 1996). In this context, the demonstration that plasma levels of quercetin can be enhanced upon supplementation or chronic administration is of particular importance. Thus, although numerous in vitro and in vivo studies provide evidence for the inhibition of carcinogenesis by quercetin, the applicability to human cancer treatment still requires further research.[1] Carrageenan-induced paw edema rat model: Male Wistar rats (200–250 g) were randomly divided into control and treatment groups. Quercetin Dihydrate was dissolved in 0.5% CMC-Na and administered orally at 25, 50, or 100 mg/kg 1 hour before carrageenan (1% w/v) injection into the hind paw. Paw thickness was measured at 1, 2, 4, and 6 hours. Serum was collected for TNF-α/IL-6 detection [1] - MCF-7 xenograft nude mouse model: 6–8-week-old BALB/c nude mice were subcutaneously injected with MCF-7 cells (2×106 cells/mouse). When tumors reached ~100 mm³, mice were treated with Quercetin Dihydrate (50, 100 mg/kg) via intraperitoneal injection every week for 5 weeks. Tumor volume was measured every 3 days. At sacrifice, tumor weight was recorded, and tissues were analyzed for p-PI3K/p-AKT and apoptotic index [2] - D-galactose-induced aging mouse model: Male ICR mice (8 weeks old) were injected with D-galactose (100 mg/kg/day, subcutaneous) for 8 weeks to induce aging. Quercetin Dihydrate (50 mg/kg/day) was administered orally during the same period. Morris water maze test evaluated cognitive function. Brain tissues were collected for SOD/CAT activity and MDA content detection [3] |
| 毒性/毒理 (Toxicokinetics/TK) |
The quercetin dihydrate oral LD50 for mice is 159 mg/kg BW[3].
In vitro toxicity: Quercetin Dihydrate (10–100 μM) had no significant cytotoxicity to normal human breast epithelial cells (MCF-10A), normal colon epithelial cells (NCM460), or primary astrocytes, with cell viability >85% at all tested concentrations [1,2,3] - In vivo toxicity: Oral/intraperitoneal administration of Quercetin Dihydrate (25–100 mg/kg/day for up to 8 weeks) in mice/rats did not cause weight loss, lethargy, or organ dysfunction. Serum ALT, AST, creatinine, and urea nitrogen levels were within normal ranges. No histological abnormalities were observed in liver, kidney, or brain tissues [1,2,3] - Plasma protein binding: Quercetin Dihydrate bound to human plasma proteins by ~90%, with no dose-dependent changes in binding affinity [2] |
| 参考文献 | |
| 其他信息 |
Ethnopharmacological relevance: Quercetin is a ubiquitous flavonoid that is present in numerous plants that are utilized in many different cultures for their nervous system and anticancer effects. To better understand the neuroprotective and antiproliferative activities of quercetin, we present a comprehensive review of the divergent actions that contribute to the ethnopharmacological profile of these plants.
Results: The pharmacological activities of quercetin that modulate antioxidation/oxidation/kinase-signaling pathways might be differentially elicited in neurons compared with malignant cells, ultimately promoting cell survival or death in a cell type- and metabolism-specific manner. Whereas the broad antioxidation and anti-inflammatory activities of quercetin are important for neuronal survival, the oxidative, kinase- and cell cycle-inhibitory, apoptosis-inducing effects of quercetin are essential for its anticancer effects. The diverse mechanistic interactions and activities of quercetin that modulate the phosphorylation state of molecules as well as gene expression would alter the interconnected and concerted intracellular signaling equilibrium, either inhibiting or strengthening survival signals. These mechanisms, which have been mainly observed in in vitro studies, cannot be easily translated into an explanation of the divergent simultaneous neuroprotective and anticancer effects observed in vivo. This is in part due to low bioavailability in plasma and in the brain, as well as the nature of the actual active molecules.
Conclusions: Numerous studies have demonstrated the beneficial effects of chronic quercetin intake, which is ethnopharmacologically meaningful, as many plants that are chronically ingested by people contain quercetin. Although quercetin and quercetin-containing plants exhibit potential as therapeutic modalities in neuropathology and in cancer, the data collectively highlight the need to elucidate issues such as bioavailability as well as its correlation with effectiveness at biomarkers in vivo. There would be an increased potentential of these plants for chemoprevention and neuropathology prevention.[1]
Environmental risk assessments characterizing potential environmental impacts of exotic weeds are more abundant and comprehensive for potential or new invaders than for widespread and well-established species such as Dalmatian (Linaria dalmatica [L.] Mill.) and yellow (L. vulgaris Mill.) toadflax. Specific effects evaluated in our assessment of environmental risks posed by yellow and Dalmatian toadflax included competitive displacement of other plant species, reservoirs of plant disease, animal and insect use, animal toxicity, human toxicity and allergenicity, erosion, and wildfire. Effect and exposure uncertainties for potential impacts of toadflax on human and ecological receptors were rated. Using publicly available information we were able to characterize ecological and human health impacts associated with toadflax, and to identify specific data gaps contributing to a high uncertainty of risk. Evidence supporting perceived negative environmental impacts of invasive toadflax was scarce.[2] Quercetin Dihydrate is a natural flavonoid found in various fruits (apples, berries), vegetables (onions, broccoli), and herbs, with antioxidant, anti-inflammatory, and anti-tumor biological activities [1,2,3] - Its core mechanisms include: 1) Scavenging ROS and enhancing antioxidant enzyme (SOD/CAT) activity to alleviate oxidative stress; 2) Inhibiting NF-κB and PI3K/AKT signaling pathways to suppress inflammation and tumor cell proliferation; 3) Inducing tumor cell apoptosis and cell cycle arrest (G2/M phase) [1,2,3] - It shows potential therapeutic applications in inflammatory diseases (rheumatoid arthritis, acute inflammation), cancers (breast cancer, colon cancer), and age-related oxidative stress disorders (cognitive decline) [1,2,3] - As a natural compound, it exhibits good biocompatibility and low toxicity, making it a promising candidate for complementary and alternative medicine [1,3] |
| 分子式 |
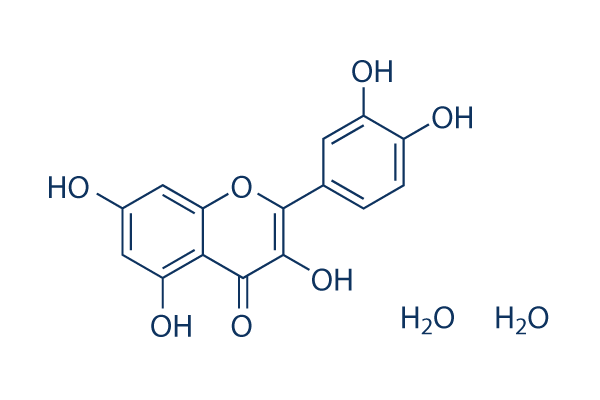
C15H10O7.2H2O
|
|---|---|
| 分子量 |
338.27
|
| 精确质量 |
338.063
|
| 元素分析 |
C, 53.26; H, 4.17; O, 42.57
|
| CAS号 |
6151-25-3
|
| 相关CAS号 |
Quercetin;117-39-5; Quercetin-d3;263711-79-1;Quercetin dihydrate;6151-25-3;Quercetin hydrate;849061-97-8; Quercetin-d5;263711-78-0;Quercetin-13C3
|
| PubChem CID |
5284452
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| 沸点 |
642.4ºC at 760 mmHg
|
| 熔点 |
>300 °C(lit.)
|
| LogP |
1.859
|
| tPSA |
149.82
|
| 氢键供体(HBD)数目 |
7
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
24
|
| 分子复杂度/Complexity |
488
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O1C(=C(C(C2=C(C([H])=C(C([H])=C12)O[H])O[H])=O)O[H])C1C([H])=C([H])C(=C(C=1[H])O[H])O[H].O([H])[H].O([H])[H]
|
| InChi Key |
GMGIWEZSKCNYSW-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C15H10O7.2H2O/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6;;/h1-5,16-19,21H;2*1H2
|
| 化学名 |
2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one;dihydrate
|
| 别名 |
Quercetin Dihydrate; 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one dihydrate; MFCD00149487; Quercetine dihydrate; Quercetin (dihydrate); 3,3',4',5,7-Pentahydroxyflavone dihydrate; DTXSID9021219;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.39 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (7.39 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9562 mL | 14.7811 mL | 29.5622 mL | |
| 5 mM | 0.5912 mL | 2.9562 mL | 5.9124 mL | |
| 10 mM | 0.2956 mL | 1.4781 mL | 2.9562 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Status | Interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01881919 | Completed | Dietary Supplement: Control Dietary Supplement: Treatment |
Gout Diabetes Hyperuricemia |
University of Leeds | February 2013 | Early Phase 1 |
Clin Cancer Res. 2013, 19(13), 3533-3544. |
PI3K inhibition-induced Rb inactivation predicts subsequent apoptosis in PTEN-deficient, ER+ breast cancer cells.Clin Cancer Res.2017 Jun 1;23(11):2795-2805. |
PI3K inhibition-induced Rb inactivation predicts subsequent apoptosis in PTEN-deficient, ER+ breast cancer cells.Clin Cancer Res.2017 Jun 1;23(11):2795-2805. |