| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|

| 体外研究 (In Vitro) |
体外活性:雷诺嗪选择性抑制晚期 I(Na),减少 [Na(+)](i) 依赖性钙超载,并减轻与心肌细胞缺血/再灌注和心力衰竭相关的心室复极和收缩力异常。在狗的左心室肌细胞中,雷诺嗪以浓度依赖性方式显着且可逆地缩短 0.5 Hz 或 0.25 Hz 刺激的肌细胞的动作电位持续时间 (APD)。 5 和 10 mM 雷诺嗪可逆地缩短抽搐收缩 (TC) 的持续时间并消除后收缩。研究发现雷诺嗪与失活状态的 I(NaL) 钠通道的结合比与静息状态的 I(NaL) 的结合更紧密。
|
|---|---|
| 体内研究 (In Vivo) |
在经历左冠状动脉前降支闭塞再灌注的大鼠中,雷诺嗪(推注 10 mg/kg 和输注 9.6 mg/kg/h;推注;持续 145 分钟;雄性 Wistar 大鼠)治疗可显着降低梗塞面积和心肌肌钙蛋白 T 释放[3]。
|
| 动物实验 |
Animal/Disease Models: Male Wistar rats (240-350 g)[3]
Doses: Bolus injection 10 mg/kg and infusion (9.6 mg/kg/h) Route of Administration: Bolus injection; for 145 minutes Experimental Results: Dramatically decreased infarct size and cardiac troponin T release in rats subjected to left anterior descending coronary artery occlusion-reperfusion. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The time to reach peak serum concentration is quite variable but has been observed to be in the range of 2-6 hours, with steady-state within 3 days. The FDA indicates a Tmax of 3-5 hours. The average steady-state Cmax is about 2600 ng/mL. Absorption of ranolazine is not significantly affected by food consumption. The bioavailability of ranolazine taken in the tablet form compared to that from a solution of ranolazine is about 76%. From the administered dose, about 3/4 of the dose is excreted renally, while 1/4 of the dose is excreted in the feces. An estimated 5% of an ingested dose is excreted as unchanged drug. The mean apparent volume of distribution of ranolazine is reported to be 53.2 L and the average steady-state volume of distribution is estimated to range from 85 to 180 L. The reported clearance rate of orally administered ranolazine is of 45 L/h when administered at a dose of 500 mg twice daily. The clearance rate of ranolazine is dose-dependent and renal impairment can increase ranolazine serum concentration by 40-50%. Ranolazine is extensively metabolized in the gut and liver and its absorption is highly variable. For example, at a dose of 1000 mg twice daily, the mean steady-state Cmax was 2600 ng/mL with 95% confidence limits of 400 and 6100 ng/mL. The pharmacokinetics of the (+) R- and (-) S-enantiomers of ranolazine are similar in healthy volunteers. ... Steady state is generally achieved within 3 days of twice-daily dosing with ranolazine. At steady state over the dose range of 500 to 1000 mg twice daily, Cmax and AUC0-t increase slightly more than proportionally to dose, 2.2- and 2.4-fold, respectively. With twice-daily dosing, the trough:peak ratio of the ranolazine plasma concentration is 0.3 to 0.6. The pharmacokinetics of ranolazine is unaffected by age, gender, or food. After oral administration of ranolazine, peak plasma concentrations of ranolazine are reached between 2 and 5 hours. After oral administration of (14)C-ranolazine as a solution, 73% of the dose is systemically available as ranolazine or metabolites. The bioavailability of ranolazine from ranolazine tablets relative to that from a solution of ranolazine is 76%. Because ranolazine is a substrate of P-gp, inhibitors of P-gp may increase the absorption of ranolazine. Food (high-fat breakfast) has no important effect on the Cmax and AUC of ranolazine. Therefore, ranolazine may be taken without regard to meals. Over the concentration range of 0.25 to 10 ug/mL, ranolazine is approximately 62% bound to human plasma proteins. It is not known whether ranolazine is distributed into milk. For more Absorption, Distribution and Excretion (Complete) data for Ranolazine (7 total), please visit the HSDB record page. Metabolism / Metabolites Ranolazine is rapidly heavily metabolized in the liver an gastrointestinal tract through the activity of the CYP3A4 enzyme with minor contributions from CYP2D6. More than 40 ranolazine metabolites have been found in plasma and more than 100 metabolites have been identified in the urine. Ranolazine and some of its metabolites are known to weakly inhibit CYP3A4. However, the activity of the metabolites of ranolazine has not been fully elucidated. Ranolazine is extensively metabolized in the intestine and liver by the cytochrome P-450 (CYP) isoenzyme system, mainly by CYP3A and, to a lesser extent, CYP2D6. In vitro studies indicate that ranolazine also is a p-glycoprotein substrate. At least 4 metabolites of ranolazine have been identified. The pharmacologic activity of these metabolites has not been fully established. Ranolazine is metabolized rapidly and extensively in the liver and intestine ... The pharmacologic activity of the metabolites has not been well characterized. After dosing to steady state with 500 mg to 1500 mg twice daily, the four most abundant metabolites in plasma have AUC values ranging from about 5 to 33% that of ranolazine... Biological Half-Life The apparent terminal half-life of ranolazine is 7 hours. ... Elimination half-life of ranolazine is 1.4-1.9 hours but is apparently prolonged, on average, to 7 hours for the ER formulation as a result of extended absorption (flip-flop kinetics). ... ... The four most abundant metabolites in plasma ... display apparent half-lives ranging from 6 to 22 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In large preregistration clinical trials, ranolazine was not associated with serum aminotransferase and alkaline phosphatase elevations during treatment and no instances of symptomatic acute liver injury were reported. Since its approval and more wide spread use, ranolazine has been linked to a single instance of mildly symptomatic, rapidly reversible, anicteric liver injury (Case 1). Immunoallergic and autoimmune features were not present. Recovery was rapid once ranolazine was discontinued. Likelihood score: D (possible rare cause of clinically apparent liver injury). Protein Binding Approximately 62% of the administered dose of ranolazine is bound to plasma proteins. Ranolazine appears to have a higher binding affinity for alpha-1 acid glycoprotein. Interactions Do not use Ranexa with strong CYP3A inhibitors, including ketoconazole, itraconazole, clarithromycin, nefazodone, nelfinavir, ritonavir, indinavir, and saquinavir. Ketoconazole (200 mg twice daily) increases average steady-state plasma concentrations of ranolazine 3.2-fold Ranolazine is a substrate and an inhibitor of the p-glycoprotein transport system; potential pharmacokinetic interactions with p-glycoprotein inhibitors (increased absorption of ranolazine). When ranolazine is co-administered with other substrates, dosage of such drugs may have to be reduced. Potential pharmacodynamic interaction (possible additive effects on QT interval). Ranolazine should be avoided in patients receiving drugs that are known to prolong the QT interval (eg, class Ia (eg, quinidine) or III (eg, dofetilide, sotalol) antiarrhythmic agents, antipsychotic agents (eg, thioridazine, ziprasidone)). Potential pharmacokinetic interaction (increased plasma ranolazine concentrations). Ranolazine should not be used with ketoconazole (a potent CYP3A inhibitor) or itraconazole. For more Interactions (Complete) data for Ranolazine (17 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Enzyme Inhibitors; Angina Pectoris/drug therapy Ranolazine is indicated for the treatment of chronic angina. Ranolazine may be used with beta-blockers, nitrates, calcium channel blockers, anti-platelet therapy, lipid-lowering therapy, ACE inhibitors, and angiotensin receptor blockers. /Included in US product label/ Drug Warnings Ranolazine is contraindicated in patients: taking strong inhibitors of CYP3A; taking inducers of CYP3A; with clinically significant hepatic impairment. Ranolazine has been shown to prolong the QT interval corrected for rate (QTc) in a dose-related manner. Although the clinical importance of QTc interval prolongation associated with ranolazine is not known, other drugs with this potential have been associated with torsades de pointes-type arrhythmias and sudden death. The mean effect on QTc interval with repeated dosing of ranolazine 1 g twice daily, at time of maximum plasma concentration (Tmax), is about 6 msec; however, in 5% of the population the prolongation of QTc interval is 15 msec. Age, weight, gender, race, heart rate, NYHA class I to IV CHF, and diabetes have no substantial effect on the relationship between ranolazine plasma concentrations and increases in QTc interval. The relationship between ranolazine concentrations and QTc remains linear over a concentration range up to fourfold greater than the concentrations produced by a ranolazine dosage of 1 g twice daily, and is not affected by changes in heart rate. The manufacturer states that ranolazine dosages exceeding 1 g twice daily should not be used. The effects of ranolazine in patients with preexisting QT interval prolongation or receiving concomitant therapy with drugs that are known to prolong the QT interval have not been established. Because of possible additive effects on the QT interval, the manufacturer states that use of ranolazine should be avoided in patients with known QT interval prolongation (including congenital long QT syndrome and uncorrected hypokalemia), known history of ventricular tachycardia, and in patients receiving drugs that prolong the QTc interval (eg, class Ia (eg, quinidine) or III (eg, dofetilide, sotalol) antiarrhythmic agents, antipsychotic agents eg, thioridazine, ziprasidone). Because the QTc-prolonging effect is increased approximately threefold in patients with hepatic dysfunction, ranolazine is contraindicated in patients with mild, moderate, or severe hepatic impairment. For more Drug Warnings (Complete) data for Ranolazine (16 total), please visit the HSDB record page. Pharmacodynamics Ranolazine exerts both antianginal and ischemic effects independent from lowering heart rate or blood pressure. It blocks IKr, the rapid portion of the delayed rectifier potassium current, and prolongs the QTc interval in a dose-dependent fashion. The Ikr is important for cardiac repolarization. Ranolazine exerts its therapeutic effects without negative chronotropic, dromotropic, or inotropic actions neither at rest, nor during exercise. |
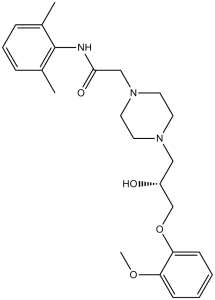
| 分子式 |
C24H33N3O4
|
|
|---|---|---|
| 分子量 |
427.54
|
|
| 精确质量 |
427.247
|
|
| CAS号 |
95635-55-5
|
|
| 相关CAS号 |
Ranolazine dihydrochloride;95635-56-6;Ranolazine-d3;1054624-77-9;Ranolazine-d5;1092804-87-9;Ranolazine-d8;1092804-88-0
|
|
| PubChem CID |
56959
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
624.1±55.0 °C at 760 mmHg
|
|
| 熔点 |
119-1200C
|
|
| 闪点 |
331.2±31.5 °C
|
|
| 蒸汽压 |
0.0±1.9 mmHg at 25°C
|
|
| 折射率 |
1.586
|
|
| LogP |
3.47
|
|
| tPSA |
74.27
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
9
|
|
| 重原子数目 |
31
|
|
| 分子复杂度/Complexity |
531
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
XKLMZUWKNUAPSZ-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C24H33N3O4/c1-18-7-6-8-19(2)24(18)25-23(29)16-27-13-11-26(12-14-27)15-20(28)17-31-22-10-5-4-9-21(22)30-3/h4-10,20,28H,11-17H2,1-3H3,(H,25,29)
|
|
| 化学名 |
N-(2,6-dimethylphenyl)-2-[4-[2-hydroxy-3-(2-methoxyphenoxy)propyl]piperazin-1-yl]acetamide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.87 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.87 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (4.87 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3390 mL | 11.6948 mL | 23.3896 mL | |
| 5 mM | 0.4678 mL | 2.3390 mL | 4.6779 mL | |
| 10 mM | 0.2339 mL | 1.1695 mL | 2.3390 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02360397 | Completed Has Results | Drug: ranolazine | Ventricular Premature Complexes Myocardial Ischemia |
Kent Hospital, Rhode Island | December 2014 | Phase 2 |
| NCT02252406 | Completed Has Results | Drug: Ranolazine Other: Placebo |
Stable Angina Metabolic Syndrome |
University of Florida | September 2015 | Phase 4 |
| NCT02239926 | Terminated Has Results | Drug: Ranolazine Drug: Placebo |
Diarrhea Predominant Irritable Bowel Syndrome |
Mayo Clinic | September 2014 | Phase 2 Phase 3 |
| NCT02133352 | Completed Has Results | Drug: Ranolazine | Pulmonary Hypertension Diastolic Left Ventricular Dysfunction |
Boston University | July 2011 | Phase 4 |
|
|
|