| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
mTOR (IC50 = 0.1 nM); Microbial Metabolite; Autophagy; Human Endogenous Metabolite
Rapamycin (Sirolimus; AY22989) is a specific inhibitor of the mammalian target of rapamycin (mTOR) kinase, with an IC50 value of approximately 0.1-0.5 nM for mTORC1 inhibition [1][3][4]. |
|---|---|
| 体外研究 (In Vitro) |
Rapamycin (Sirolimus; AY22989)抑制 HEK293 细胞中的内源性 mTOR 活性,IC50 约为 0.1 nM,比 iRap 和 AP21967 更有效,IC50 分别为约 5 nM 和约 10 nM。 [1] Rapamycin (Sirolimus; AY22989)/雷帕霉素治疗会导致酿酒酵母中严重的 G1/S 细胞周期停滞,并将翻译起始抑制至低于对照的 20% 水平。 [2] 雷帕霉素对 U373-MG 细胞几乎没有活性,IC50 > 25 M,尽管对 mTOR 信号传导的抑制具有类似的影响。 Rapamycin 以剂量依赖性方式显着降低 T98G 和 U87-MG 的细胞活力。通过抑制 mTOR 的活性,雷帕霉素 (100 nM) 会导致雷帕霉素敏感的 U87-MG 和 T98G 细胞发生 G1 期阻滞和自噬,但不会导致细胞凋亡。 [3]
用免疫抑制剂Rapamycin (Sirolimus; AY22989)/雷帕霉素处理或耗尽雷帕霉素TOR1和TOR2靶点的酿酒酵母细胞在细胞周期的早期G1期停止生长。TOR功能的丧失还会导致翻译起始的早期抑制,并诱导饥饿细胞进入静止期(G0)的其他一些生理变化。G1细胞周期蛋白mRNA的翻译控制通过替换UBI4 5'先导区而改变(UBI4通常在饥饿条件下被翻译),抑制雷帕霉素诱导的G1阻滞并给予饥饿敏感性。这些结果表明,翻译起始的阻滞是TOR功能丧失的直接后果,也是G1停滞的原因。我们提出,tor,两个相关的磷脂酰肌醇激酶同源物,是激活eif - 4e依赖性蛋白合成的新信号通路的一部分,因此,在对营养可用性的反应中,G1进程。这种途径可能构成一个检查点,在缺乏营养的情况下阻止早期G1进展和生长。[2] 哺乳动物雷帕霉素靶蛋白(mTOR)是磷脂酰肌醇3-激酶(PI3K)/蛋白激酶B (Akt)信号通路的下游效应分子,是恶性胶质瘤细胞增殖的中枢调节剂。因此,靶向mTOR信号被认为是治疗恶性胶质瘤的一种很有前景的方法。然而,选择性mTOR抑制剂Rapamycin (Sirolimus; AY22989)/雷帕霉素对恶性胶质瘤细胞的细胞毒性作用机制尚不清楚。因此,本研究的目的是阐明雷帕霉素如何对恶性胶质瘤细胞发挥其细胞毒性作用。我们发现雷帕霉素通过抑制mTOR的功能诱导雷帕霉素敏感的恶性胶质瘤U87-MG和T98G细胞自噬,而不是凋亡。相比之下,在雷帕霉素耐药的U373-MG细胞中,雷帕霉素的抑制作用很小,尽管mTOR下游分子p70S6激酶的磷酸化被显著抑制。有趣的是,PI3K抑制剂LY294002和Akt抑制剂UCN-01(7-羟基脲孢素)均通过刺激诱导自噬,使U87-MG和T98G细胞以及U373-MG细胞对雷帕霉素增敏。在肿瘤细胞中强制表达活性Akt抑制LY294002或UCN-01的联合作用,而Akt的显性阴性表达则足以增加肿瘤细胞对雷帕霉素的敏感性。 - 胶质瘤细胞自噬诱导:在恶性胶质瘤细胞系(如U87-MG)中,雷帕霉素 (10 nM) 诱导自噬,表现为LC3-II蛋白水平升高和自噬体形成。与磷脂酰肌醇3-激酶 (PI3K) 抑制剂(如LY294002, 10 μM)联合处理可协同增强雷帕霉素诱导的自噬,导致细胞活力较单药治疗显著降低 [3]。 - mTORC1抑制:雷帕霉素 (0.1-10 nM) 在体外有效抑制mTORC1活性,表现为多种细胞类型中下游靶点(如S6K1和4E-BP1)的磷酸化水平降低 [1][3][4]。 |
| 体内研究 (In Vivo) |
体内Rapamycin (Sirolimus; AY22989)/雷帕霉素治疗可特异性阻断 mTOR 下游的靶点,例如 p70S6K 的磷酸化和激活以及 PHAS-1/4E-BP1 对 eIF4E 的抑制作用的释放,从而完全阻断跖肌重量的肥大性增加和纤维尺寸。[4]短期雷帕霉素治疗,即使是最低剂量 0.16 mg/kg,也会导致 p70S6K 活性的深度抑制,这与 Eker 肾肿瘤的肿瘤细胞死亡和坏死增加相关。 [5] 通过降低 VEGF 产生并阻止 VEGF 诱导的内皮细胞信号传导,雷帕霉素可抑制 CT-26 异种移植模型中的血管生成和转移性肿瘤生长。 [6] 4 mg/kg/天的雷帕霉素治疗可显着降低 C6 异种移植物中的肿瘤血管通透性和肿瘤生长。 [7]
骨骼肌通过调节纤维大小的未知机制来适应工作负荷的变化。Akt/mTOR(哺乳动物雷帕霉素靶蛋白)和钙调磷酸酶/NFAT(活化T细胞核因子)这两种信号通路在体内骨骼肌肥大和萎缩模型中参与肌肉肥大的作用基于体外研究结果。Akt/mTOR通路在肌肉肥大时上调,在肌肉萎缩时下调。此外,Rapamycin (Sirolimus; AY22989)/雷帕霉素是一种选择性mTOR阻滞剂,在所有模型中都能阻断肥厚,而不引起对照肌肉萎缩。相比之下,钙调神经磷酸酶途径在体内肥厚过程中不被激活,钙调神经磷酸酶抑制剂、环孢素A和FK506并没有钝化肥厚。最后,遗传激活Akt/mTOR通路足以在体内引起肥大并防止萎缩,而遗传阻断该通路则会阻断体内肥大。我们得出的结论是,Akt/mTOR通路及其下游靶点p70S6K和phase -1/ 4e - bp1的激活是调控骨骼肌纤维大小的必要条件,Akt/mTOR通路的激活可以对抗废用引起的肌肉萎缩。 小鼠肌肉萎缩预防:在 hindlimb unloading 诱导的小鼠肌肉萎缩模型中,雷帕霉素(1 mg/kg/ 天,腹腔注射)处理 14 天显著防止肌肉萎缩。与溶媒处理对照组相比,处理组小鼠的胫前肌和腓肠肌重量增加 20-30%,横截面积增大 15-25%。此外,处理后肌肉中萎缩相关基因(Atrogin-1 和 MuRF-1)的表达降低 40-50% [4]。 骨骼肌肥大调控:在 IGF-1 诱导的小鼠骨骼肌肥大模型中,雷帕霉素(1 mg/kg/ 天,腹腔注射)处理 7 天可抑制肥大反应,与单独 IGF-1 处理相比,肌肉质量增加减少 25-35%。这与肌肉组织中 S6K1 和 4E-BP1 的磷酸化水平降低相关 [4]。 |
| 酶活实验 |
HEK293 细胞以 2-2.5×105 个细胞/孔铺在 12 孔板中,并在 DMEM 中血清饥饿 24 小时。Rapamycin (Sirolimus; AY22989)/雷帕霉素 (0.05–50 nM) 在 37 °C 下以递增浓度给予细胞 15 分钟。在 37°C 下花费 30 分钟添加终浓度为 20% 的血清。细胞裂解物在裂解后通过 SDS-PAGE 分离。将已解析的蛋白质转移到聚偏二氟乙烯膜上,并使用对 p70 S6 激酶的 Thr-389 具有磷酸特异性的一抗进行免疫印迹。使用 ImageQuant 和 KaleidaGr 进行数据分析。[1]
雷帕霉素/Rapamycin (Sirolimus; AY22989)是一种免疫抑制药物,同时结合12 kda的FK506-和雷帕霉素结合蛋白(FKBP12,或FKBP)和哺乳动物雷帕霉素靶蛋白(mTOR)激酶的FKBP-雷帕霉素结合(FRB)结构域。所得到的三元配合物已被用于有条件地干扰蛋白质功能,其中一种方法涉及通过其错定位干扰感兴趣的蛋白质。我们合成了两个在FRB结合界面C-16位置具有大取代基的雷帕霉素衍生物,并使用酵母的三杂交实验对这些衍生物进行了FRB突变体文库的筛选。几种FRB突变体对一种雷帕霉素衍生物有反应,其中20种突变体在哺乳动物细胞中得到进一步表征。将对配体反应最灵敏的突变体与黄色荧光蛋白融合,并测量存在和不存在配体时的荧光水平,以确定融合蛋白的稳定性。在没有雷帕霉素衍生物的情况下,野生型和突变型FRB结构域的表达水平很低,而在配体处理后,表达水平上升到10倍。对合成的雷帕霉素衍生物进行定量质谱分析,发现其中一种化合物含有污染雷帕霉素。此外,未受污染的类似物保留了抑制mTOR的能力,尽管相对于雷帕霉素的效力有所减弱。在使用这些系统时,应考虑野生型FRB和FRB突变体所显示的配体依赖性稳定性以及雷帕霉素衍生物的抑制潜力和纯度,这是潜在的混淆实验变量。[1] mTOR激酶活性测定:将重组mTOR激酶与ATP和合成肽底物在雷帕霉素 (0.01-100 nM) 存在下孵育。反应通过加入SDS-PAGE上样缓冲液终止,磷酸化产物用磷酸特异性抗体通过免疫印迹检测。雷帕霉素 抑制mTOR激酶活性的IC50为0.1-0.5 nM [1][3][4]。 |
| 细胞实验 |
将细胞暴露于不同浓度的Rapamycin (Sirolimus; AY22989)/雷帕霉素中 72 小时。为了评估细胞活力,通过胰蛋白酶消化收集细胞,用台盼蓝染色,并对每孔中的活细胞进行计数。为了测定细胞周期,将细胞用胰蛋白酶消化,用 70% 乙醇固定,并使用流式细胞术试剂套件用碘化丙啶染色。使用 FACScan 流式细胞仪和 CellQuest 软件分析样品的 DNA 含量。对于细胞凋亡检测,使用 ApopTag 细胞凋亡检测试剂盒通过末端脱氧核苷酸转移酶介导的 dUTP 缺口末端标记 (TUNEL) 技术对细胞进行染色。为了检测酸性囊泡细胞器 (AVO) 的发育,将细胞用吖啶橙 (1 μg/mL) 染色 15 分钟,并在荧光显微镜下检查。为了量化 AVO 的发育,将细胞用吖啶橙 (1 μg/mL) 染色 15 分钟,用胰蛋白酶-EDTA 从板中取出,并使用 FACScan 流式细胞仪和 CellQuest 软件进行分析。为了分析自噬过程,将细胞与 0.05 mM monodansylcadaverine 在 37 °C 下孵育 10 分钟,然后在荧光显微镜下观察。
细胞活力测定[3] 测定Rapamycin (Sirolimus; AY22989)/雷帕霉素和Rapamycin (Sirolimus; AY22989) + LY294002或UCN-01作用于肿瘤细胞,我们测定了治疗后的细胞活力。我们使用了先前描述的台盼蓝染料排除试验。采集呈指数生长的肿瘤细胞,以每孔5 × 103个细胞(0.1 mL)接种于96孔平底板,37℃孵育过夜。然后将细胞加雷帕霉素或不加雷帕霉素或雷帕霉素加LY294002或UCN-01孵育72小时。胰蛋白酶化收集细胞后,用台盼蓝染色,计数每孔活细胞数。未经处理的细胞(对照组)存活率为100%。根据处理细胞的平均细胞活力计算存活分数。[3] - 胶质瘤细胞活力测定:恶性胶质瘤细胞用雷帕霉素 (1-100 nM) 单独或与PI3K抑制剂 (1-10 μM) 联合处理48小时。使用MTT法评估细胞活力。雷帕霉素 单独处理在10 nM时使细胞活力降低30-50%,而联合处理导致60-80%的降低 [3]。 - 骨骼肌细胞肥大测定:在C2C12成肌细胞中,雷帕霉素 (10 nM) 抑制胰岛素样生长因子1 (IGF-1) 诱导的肥大,表现为细胞大小减小和肥大标志物(如MyoD、肌生成素)表达降低 [4]。 |
| 动物实验 |
Athymic Nu/Nu mice inoculated subcutaneously with VEGF-A-expressing C6 rat glioma cells
~4 mg/kg/day Injection i.p. Drug administration in vivo.[4] Animals were randomized to treatment or vehicle groups so that the mean starting body weights of each group were equal. Drug treatment began on the day of surgery or on the first day of reloading after the 14-day suspension. Rapamycin was delivered once daily by intraperitoneal injection at a dose of 1.5 mg kg−1, dissolved in 2% carboxymethylcellulose. CsA was delivered once daily by subcutaneous injection at a dose of 15 mg kg−1, dissolved in 10% methanol and olive oil. FK506 was delivered once daily via subcutaneous injection at a dose of 3 mg kg−1, dissolved in 10% ethanol, 10% cremophor and saline.[4] Muscle Atrophy Prevention in Mice: Rapamycin (1 mg/kg/day, intraperitoneal injection) was administered to mice subjected to hindlimb unloading to induce muscle atrophy. Treatment significantly prevented muscle wasting, as measured by muscle weight and cross-sectional area of tibialis anterior and gastrocnemius muscles. Rapamycin also reduced the expression of atrophy-related genes (e.g., Atrogin-1, MuRF-1) [4]. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
In adult renal transplant patients with low- to moderate-immunologic risk, oral administration of 2 mg sirolimus led to a Cmax of 14.4 ± 5.3 ng/mL for oral solution and 15.0 ± 4.9 ng/mL for oral tablets. The tmax was 2.1 ± 0.8 hours for oral solution and 3.5 ± 2.4 hours for oral tablets. In healthy subjects, the tmax is one hour. In a multi-dose study, steady-state was reached six days following repeated twice-daily administration without an initial loading dose, with the average trough concentration of sirolimus increased approximately 2- to 3-fold. It is suspected that a loading dose of three times the maintenance dose will provide near steady-state concentrations within one day in most patients. The systemic availability of sirolimus is approximately 14%. In healthy subjects, the mean bioavailability of sirolimus after administration of the tablet is approximately 27% higher relative to the solution. Sirolimus tablets are not bioequivalent to the solution; however, clinical equivalence has been demonstrated at the 2 mg dose level. Sirolimus concentrations, following the administration of Rapamune Oral Solution to stable renal transplant patients, are dose-proportional between 3 and 12 mg/m2. Following oral administration of [14C] sirolimus in healthy subjects, about 91% of the radioactivity was recovered from feces and only 2.2% of the radioactivity was detected in urine. Some of the metabolites of sirolimus are also detectable in feces and urine. The mean (± SD) blood-to-plasma ratio of sirolimus was 36 ± 18 L in stable renal allograft patients, indicating that sirolimus is extensively partitioned into formed blood elements. The mean volume of distribution (Vss/F) of sirolimus is 12 ± 8 L/kg. In adult renal transplant patients with low- to moderate-immunologic risk, oral administration of 2 mg sirolimus led to oral clearance of 173 ± 50 mL/h/kg for oral solution and 139 ± 63 mL/h/kg for oral tablets. Following administration of /Sirolimus/ Oral Solution, sirolimus is rapidly absorbed, with a mean time-to-peak concentration (t max ) of approximately 1 hour after a single dose in healthy subjects and approximately 2 hours after multiple oral doses in renal transplant recipients. The systemic availability of sirolimus was estimated to be approximately 14% after the administration of /Sirolimus/ Oral Solution. The mean bioavailability of sirolimus after administration of the tablet is about 27% higher relative to the oral solution. In 22 healthy volunteers receiving Rapamune Oral Solution, a high-fat meal altered the bioavailability characteristics of sirolimus. Compared with fasting, a 34% decrease in the peak blood sirolimus concentration (C max ), a 3.5-fold increase in the time-to-peak concentration (t max ), and a 35% increase in total exposure (AUC) was observed. After administration of Rapamune Tablets and a high-fat meal in 24 healthy volunteers, C max , t max , and AUC showed increases of 65%, 32%, and 23%, respectively. Absorption: Rapid, from the gastrointestinal tract. Bioavailability is approximately 14%. Rate of absorption is decreased in the presence of a high-fat diet. The rate and extent of absorption is reduced in black patients. The mean (+/- SD) blood-to-plasma ratio of sirolimus was 36 +/- 17.9 in stable renal allograft recipients, indicating that sirolimus is extensively partitioned into formed blood elements. The mean volume of distribution of sirolimus is 12 +/- 7.52 L/kg. Sirolimus is extensively bound (approximately 92%) to human plasma proteins. In man, the binding of sirolimus was shown mainly to be associated with serum albumin (97%), (alpha) 1 -acid glycoprotein, and lipoproteins. For more Absorption, Distribution and Excretion (Complete) data for SIROLIMUS (7 total), please visit the HSDB record page. Metabolism / Metabolites Sirolimus undergoes extensive metabolism in the intestinal wall and liver. Sirolimus is primarily metabolized by O-demethylation and/or hydroxylation via CYP3A4 to form seven major metabolites, including hydroxy, demethyl, and hydroxydemethyl metabolites, which are pharmacologically inactive. Sirolimus also undergoes counter-transport from enterocytes of the small intestine into the gut lumen. Sirolimus is a substrate for both cytochrome P450 IIIA4 (CYP3A4) and P-glycoprotein. Sirolimus is extensively metabolized by O-demethylation and/or hydroxylation. Seven major metabolites, including hydroxy, demethyl, and hydroxydemethyl, are identifiable in whole blood. Some of these metabolites are also detectable in plasma, fecal, and urine samples. Glucuronide and sulfate conjugates are not present in any of the biologic matrices. Biotransformation: Hepatic, extensive, by cytochrome p450 3A enzymes. Major metabolites include hydroxysirolimus, demethylsirolimus, and hydroxydemethyl-sirolimus. ... After incubation of sirolimus with human and pig small intestinal microsomes, five metabolites were detected using high performance liquid chromatography/electrospray-mass spectrometry: hydroxy, dihydroxy, trihydroxy, desmethyl and didesmethyl sirolimus. The same metabolites were generated by human liver microsomes and pig small intestinal mucosa in the Ussing chamber. Anti-CYP3A antibodies, as well as the specific CYP3A inhibitors troleandomycin and erythromycin, inhibited small intestinal metabolism of sirolimus, confirming that, as in the liver, CYP3A enzymes are responsible for sirolimus metabolism in the small intestine. ... Sirolimus has known human metabolites that include 16-O-Desmethylsirolimus, 39-O-Desmethylsirolimus, 24-Hydroxy-sirolimus, 11-Hydroxy-sirolimus, 25-Hydroxy-sirolimus, 46-Hydroxy-sirolimus, and 12-Hydroxy-sirolimus. Biological Half-Life The mean ± SD terminal elimination half-life (t½) of sirolimus after multiple dosing in stable renal transplant patients was estimated to be about 62 ± 16 hours. The drug has an elimination half life of 57-63 hours in kidney transplant recipients. - Oral Bioavailability: In preclinical studies, Rapamycin exhibited low oral bioavailability (~15-20%) due to extensive first-pass metabolism in the liver and intestine [1][3]. - Half-Life: The plasma half-life of Rapamycin in mice and rats was approximately 6-12 hours after intravenous administration [1][3]. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Serum enzyme elevations occur in a proportion of patients taking sirolimus, but the abnormalities are usually mild, asymptomatic and self-limiting, rarely requiring dose modification or discontinuation. Rare instances of cholestatic hepatitis have been reported with sirolimus use, but the clinical features of the clinically apparent liver injury due to this agent have not been well defined. Most published cases of liver injury attributed to sirolimus occurred in patients exposed to other potentially hepatotoxic agents or who have other underlying possible causes of the abnormalities such as sepsis, cancer or parenteral nutrition. Hepatic artery thrombosis has been reported to be more common with sirolimus therapy after liver transplantation, but this association is still controversial. Likelihood score: C (probable rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Because almost no information is available on the use of oral sirolimus during breastfeeding, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. Sirolimus is undetectable in the bloodstream after application to the skin, so use of topical sirolimus is unlikely to affect a nursing infant. Avoid application to the nipple area and ensure that the infant's skin does not come into direct contact with the areas of skin that have been treated. ◉ Effects in Breastfed Infants One infant was reported breastfed (extent not stated) during maternal therapy with sirolimus, tacrolimus and prednisone in unspecified dosages following a kidney-pancreas transplant. The authors who followed the mother knew of no serious side effects in the infant. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Sirolimus is 92% bound to human plasma proteins, mainly serum albumin (97%), α1-acid glycoprotein, and lipoproteins. Interactions Because St. John's wort (hypericum perforatum) induces the activity of CYP3A4 and P-glycoprotein and sirolimus is a substrate of both, concurrent use of St. John's wort with sirolimus may result in decreased sirolimus concentrations. /Concurrent use of sirolimus with tacrolimus/ may cause excess mortality, graft loss and hepatic artery thrombosis (HAT) in liver transplant patients, most cases of HAT occured within 30 days post-transplantation. /Antibiotics such as: rifabutin or rifapentine; and anticonvulsants such as: carbamazepine, phenobarbital, or phenytoin/ may decrease sirolimus concentrations due to cytochrome p450 3A4 (CYP3 A4) isoenzyme induction. Significant increases in sirolimus clearance occur when administered with rifampin due to CYP3A4 induction by rifampin; an alternative antibacterial agent with less enzyme induction potential should be considered. For more Interactions (Complete) data for SIROLIMUS (11 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Mouse ip 600 mg/kg LD50 Mouse oral >2,500 mg/kg |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Sirolimus is indicated for the prevention of rejection of transplanted kidney allografts. It is recommended that sirolimus be used in a regimen with cyclosporine and corticosteroids. /Included in US product labeling/ Long-term results after percutaneous coronary intervention in the treatment of chronic total coronary occlusions is hindered by a significant rate of restenosis and reocclusion. In the treatment of relatively simple nonocclusive lesions, sirolimus-eluting stents have shown dramatically reduced restenosis rates compared with bare metal stents, but whether these results are more widely applicable is unknown. ... The use of sirolimus-eluting stents in the treatment of chronic total coronary occlusions is associated with a reduction in the rate of major adverse cardiac events and restenosis compared with bare metal stents. Chronic renal failure triggered by calcineurin inhibitor (CNI)-based immunosuppression is a common complication after cardiac transplantation. Sirolimus and mycophenolate mofetil (MMF) are 2 newer immunosuppressive agents with no documented nephrotoxic side effects. This case report describes a patient with ongoing chronic renal failure 10 months after cardiac transplantation on cyclosporine-based immunosuppressive therapy. Conversion of the immunosuppressive regimen from cyclosporine to sirolimus and MMF resulted in freedom from acute rejection, excellent cardiac graft function and consistently improved renal function. This case illustrates the beneficial potential of sirolimus and MMF as CNI-free and safe long-term immunosuppression in a patient with chronic renal failure after heart transplantation. Drug Warnings /BOXED WARNING/ IMMUNOSUPPRESSION, USE IS NOT RECOMMENDED IN LIVER OR LUNG TRANSPLANT PATIENTS. Increased susceptibility to infection and the possible development of lymphoma and other malignancies may result from immunosuppression Increased susceptibility to infection and the possible development of lymphoma may result from immunosuppression. Only physicians experienced in immunosuppressive therapy and management of renal transplant patients should use Rapamune. Patients receiving the drug should be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources. The physician responsible for maintenance therapy should have complete information requisite for the follow-up of the patient. The safety and efficacy of Rapamune (sirolimus) as immunosuppressive therapy have not been established in liver or lung transplant patients, and therefore, such use is not recommended. Liver Transplantation - Excess Mortality, Graft Loss, and Hepatic Artery Thrombosis (HAT): The use of Rapamune in combination with tacrolimus was associated with excess mortality and graft loss in a study in de novo liver transplant patients. Many of these patients had evidence of infection at or near the time of death. In this and another study in de novo liver transplant patients, the use of Rapamune in combination with cyclosporine or tacrolimus was associated with an increase in HAT; most cases of HAT occurred within 30 days post-transplantation and most led to graft loss or death. Lung Transplantation - Bronchial Anastomotic Dehiscence: Cases of bronchial anastomotic dehiscence, most fatal, have been reported in de novo lung transplant patients when Rapamune has been used as part of an immunosuppressive regimen. Grapefruit juice may inhibit CYP 3A4 enzymes, leading to decreased metabolism of sirolimus; must not be taken with or used to dilute sirolimus. Cases of bronchial anastomotic dehiscence, most of which were fatal, have been reported in de novo lung transplant patients who received sirolimus in combination with other immunosuppressants. Because safety and efficacy of sirolimus as immunosuppressive therapy in lung transplant patients have not been established, such use in not recommended by the manufacturer. Use of sirolimus in combination with other immunosuppressants (i.e., cyclosporine, tacrolimus) has been associated with an increased risk on hepatic artery thrombosis, graft loss, and death in de novo liver transplant recipients. Because safety and efficacy of sirolimus as immunosuppressive therapy in liver transplant patients have not been established, such use is not recommended by the manufacturer. For more Drug Warnings (Complete) data for SIROLIMUS (27 total), please visit the HSDB record page. Pharmacodynamics Sirolimus is an immunosuppressant drug with antifungal and antitumour effects. In animal models, sirolimus prolonged allograft survival following various organ transplants and reversed an acute rejection of heart and kidney allografts in rats. Upon oral administration of 2 mg/day and 5 mg/day, sirolimus significantly reduced the incidence of organ rejection in low- to moderate-immunologic risk renal transplant patients at six months following transplantation compared with either azathioprine or placebo. In some studies, the immunosuppressive effect of sirolimus lasted up to six months after discontinuation of therapy: this tolerization effect is alloantigen-specific. Sirolimus potently inhibits antigen-induced proliferation of T cells, B cells, and antibody production. In rodent models of autoimmune disease, sirolimus suppressed immune-mediated events associated with systemic lupus erythematosus, collagen-induced arthritis, autoimmune type I diabetes, autoimmune myocarditis, experimental allergic encephalomyelitis, graft-versus-host disease, and autoimmune uveoretinitis. - Mechanism of Action: Rapamycin binds to FKBP12, forming a complex that inhibits mTORC1 by blocking its kinase activity. This leads to suppression of protein synthesis, induction of autophagy, and inhibition of cell growth and proliferation [1][3][4]. - Clinical Applications: Rapamycin is approved for immunosuppression in organ transplantation and treatment of certain cancers. It has also shown promise in preclinical models for preventing muscle atrophy and treating neurodegenerative diseases [1][3][4]. - Side Effects: Common side effects of Rapamycin include immunosuppression, hyperlipidemia, and hyperglycemia. Long-term use may increase the risk of infections and certain cancers [1][3][4]. |
| 分子式 |
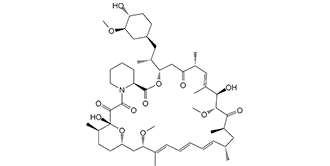
C51H79NO13
|
|---|---|
| 分子量 |
914.18
|
| 精确质量 |
913.555
|
| 元素分析 |
C, 67.01; H, 8.71; N, 1.53; O, 22.75
|
| CAS号 |
53123-88-9
|
| 相关CAS号 |
Rapamycin;53123-88-9
|
| PubChem CID |
5284616
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
973.0±75.0 °C at 760 mmHg
|
| 熔点 |
183-185°C
|
| 闪点 |
542.3±37.1 °C
|
| 蒸汽压 |
0.0±0.6 mmHg at 25°C
|
| 折射率 |
1.551
|
| LogP |
3.54
|
| tPSA |
195.43
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
13
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
65
|
| 分子复杂度/Complexity |
1760
|
| 定义原子立体中心数目 |
15
|
| SMILES |
O(C([H])([H])[H])[C@@]1([H])[C@@]([H])(C([H])([H])C([H])([H])[C@@]([H])(C([H])([H])[C@@]([H])(C([H])([H])[H])[C@]2([H])C([H])([H])C([C@@]([H])(C([H])=C(C([H])([H])[H])[C@]([H])([C@]([H])(C([C@]([H])(C([H])([H])[H])C([H])([H])[C@]([H])(C([H])([H])[H])C([H])=C([H])C([H])=C([H])C([H])=C(C([H])([H])[H])[C@]([H])(C([H])([H])[C@]3([H])C([H])([H])C([H])([H])[C@@]([H])(C([H])([H])[H])[C@@](C(C(N4C([H])([H])C([H])([H])C([H])([H])C([H])([H])[C@@]4([H])C(=O)O2)=O)=O)(O[H])O3)OC([H])([H])[H])=O)OC([H])([H])[H])O[H])C([H])([H])[H])=O)C1([H])[H])O[H] |c:35,66,70,t:62|
|
| InChi Key |
QFJCIRLUMZQUOT-PYYJPVDBSA-N
|
| InChi Code |
InChI=1S/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43+,44?,46-,47+,51-/m1/s1
|
| 化学名 |
(3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34, 34a-hexadecahydro-9,27-dihydroxy-3-[(1R)-2-[(1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylethyl]-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-23,27-epoxy-3H-pyrido[2,1-c][1,4] oxaazacyclohentriacontine-1,5,11,28,29 (4H,6H,31H)-pentone
|
| 别名 |
AY 22989; AY22989; AY-22989; NSC-2260804; RAPA; RAP; RPM; SLM; AY 22989; SILA 9268A; WY090217; WY-090217; WY 090217; C07909; D00753; I 2190A; I-2190A; I2190A; NSC 226080; Rapamune
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
体内配方 1: 2% DMSO + 30% PEG 300+5% Tween 80+ddH2O: 5 mg/mL; 悬浊液
体内配方 2: 0.5% CMC-Na + 1%Tween-80 in Saline water: 1.98 mg/mL (2.17 mM); 悬浊液 体内配方 3:10% DMSO + 90% Corn Oil: ≥ 2.08 mg/mL (2.28 mM); 澄清溶液 体内配方 4:10% EtOH + 40% PEG300 + 5% Tween-80 + 45% Saline: ≥ 2.5 mg/mL (2.73 mM); 悬浊液 体内配方 5:10% EtOH + 90% (20% SBE-β-CD in Saline): 2.5 mg/mL (2.73 mM); 悬浊液 体内配方 6:10% EtOH + 90% Corn Oil: ≥ 2.5 mg/mL (2.73 mM); 悬浊液 体内配方 7:10% DMSO + 40% PEG300 + 5% Tween-80 + 45% Saline: ≥ 2.08 mg/mL (2.28 mM); 澄清溶液 体内配方 8:10% DMSO + 90% (20% SBE-β-CD in Saline): 2.08 mg/mL (2.28 mM); 悬浊液 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.0939 mL | 5.4694 mL | 10.9388 mL | |
| 5 mM | 0.2188 mL | 1.0939 mL | 2.1878 mL | |
| 10 mM | 0.1094 mL | 0.5469 mL | 1.0939 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
CD40-L Blockade for Prevention of Acute Graft-Versus-Host Disease
CTID: NCT03605927
Phase: Phase 1 Status: Completed
Date: 2024-11-27
|
 |
 |