| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|

| 靶点 |
CYP3A4; HIV
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:Ritonavir 是 CYP3A4 介导的睾酮 6β-羟基化的非常有效的抑制剂,平均 Ki 为 19 nM,并且还抑制甲苯磺丁脲羟基化,IC50 为 4.2 μM。 Ritonavir被发现是CYP3A介导的生物转化的有效抑制剂(硝苯地平氧化,IC50为0.07 mM,17α-乙炔雌二醇2-羟基化,IC50为2 mM;特非那定羟基化,IC50为0.14 mM)。 Ritonavir 还被发现是 CYP2D6 (IC50 = 2.5 mM) 和 CYP2C9/10 (IC50 = 8.0 mM) 介导的反应的抑制剂。利托那韦可增加未感染的人 PBMC 培养物中的细胞活力。 Ritonavir 显着降低 PBMC 对细胞凋亡的敏感性,这与较低水平的 caspase-1 表达相关,减少膜联蛋白 V 染色,并降低未感染的人 PBMC 培养物中的 caspase-3 活性。 Ritonavir 在无毒浓度下以时间和剂量依赖性方式抑制 PBMC 和单核细胞诱导肿瘤坏死因子 (TNF) 产生。 Ritonavir 抑制 p-糖蛋白介导的沙奎那韦挤出,IC50 为 0.2 μM,表明利托那韦对 p-糖蛋白具有高亲和力。 Ritonavir 有效抑制 ABT-378 的人肝微粒体代谢,Ki 为 13 nM。 Ritonavir 与 ABT-378 联合(比例为 3:1 和 29:1)可抑制 CYP3A(IC50 = 1.1 和 4.6 μM),但不如 Ritonavir(IC50 = 0.14 μM)有效。激酶测定:Ritonavir (ABT 538) 是 CYP3A4 介导的睾酮 6β-羟基化的抑制剂,平均 Ki 为 19 nM,并且还抑制甲苯磺丁脲羟基化,IC50 为 4.2 μM。 Ritonavir (ABT 538) 被发现是 CYP3A 介导的生物转化的有效抑制剂(硝苯地平氧化,IC50 为 0.07 mM,17α-乙炔雌二醇 2-羟基化,IC50 为 2 mM;特非那定羟基化,IC50 为 0.14 mM)。 Ritonavir 也是 CYP2D6 (IC50=2.5 mM) 和 CYP2C9/10 (IC50=8.0 mM) 介导的反应的抑制剂。细胞测定:利托那韦可增加未感染的人 PBMC 培养物中的细胞活力。 Ritonavir 显着降低 PBMC 对细胞凋亡的敏感性,这与较低水平的 caspase-1 表达相关,减少膜联蛋白 V 染色,并降低未感染的人 PBMC 培养物中的 caspase-3 活性。 Ritonavir 在无毒浓度下以时间和剂量依赖性方式抑制 PBMC 和单核细胞诱导肿瘤坏死因子 (TNF) 产生。 Ritonavir 抑制 p-糖蛋白介导的沙奎那韦挤出,IC50 为 0.2 μM,表明利托那韦对 p-糖蛋白具有高亲和力。 Ritonavir 有效抑制 ABT-378 的人肝微粒体代谢,Ki 为 13 nM。 Ritonavir 与 ABT-378 联合(比例为 3:1 和 29:1)可抑制 CYP3A(IC50=1.1 和 4.6 μM),但不如 Ritonavir(IC50=0.14 μM)有效。
|
| 体内研究 (In Vivo) |
PAXLOVID™(Nirmatrevir与利托那韦的联合包装)已被批准用于治疗2019冠状病毒病(新冠肺炎)。该实验的目的是使用超高效液相色谱-串联质谱法(UPLC-MS/MS)创建一种准确直接的分析方法,同时定量大鼠血浆中的尼马替韦和利托那韦,并研究这些药物在大鼠体内的药代动力学特征。使用乙腈进行蛋白质沉淀后,使用超高效液相色谱法(UPLC)分离尼马特洛韦、利托那韦和内标(IS)洛匹那韦。这种分离是通过使用具有二元梯度洗脱的反相柱,使用由乙腈和0.1%甲酸水溶液组成的流动相实现的。使用多反应监测(MRM)技术,在正电喷雾电离模式下检测分析物。在血浆样本中,尼马替韦和利托那韦的校准范围分别为2.0-10000 ng/mL和1.0-5000 ng/mL,观察到良好的线性关系。尼马特韦和利托那韦的定量下限分别为2.0 ng/mL和1.0 ng/mL。两种药物的日间和日间精度均低于15%,准确率在-7.6%至13.2%之间。分析物的提取回收率高于90.7%,没有明显的基质效应。同样,在不同条件下,稳定性满足分析方法的要求。这种UPLC-MS/MS方法的特点是能够准确和精确地定量血浆中的尼马特韦和利托那韦,可有效用于大鼠体内药代动力学研究[8]。
|
| 酶活实验 |
Ritonavir (ABT 538) 是一种由 CYP3A4 介导的睾酮 6β-羟基化抑制剂,平均 Ki 为 19 nM。它对甲苯磺丁脲羟基化的 IC50 为 4.2 μM。研究发现,利托那韦 (ABT 538) 是 CYP3A 介导的生物转化的强抑制剂(硝苯地平氧化和 17α-乙炔雌二醇 2-羟基化的 IC50 值分别为 0.07 mM、2 mM 和 0.14 mM)。 CYP2D6 (IC50=2.5 mM) 和 CYP2C9/10 (IC50=8.0 mM) 介导的反应抑制剂包括利托那韦。
|
| 细胞实验 |
在未感染的人外周血单核细胞中,利托那韦可增加细胞活力。在未感染的人 PBMC 培养物中,利托那韦显着降低 PBMC 对细胞凋亡的敏感性,这与 caspase-1 表达水平降低、膜联蛋白 V 染色减少以及 caspase-3 活性降低相关。在无毒浓度下,利托那韦以时间和剂量依赖性方式抑制单核细胞和 PBMC 产生肿瘤坏死因子 (TNF) 的诱导。利托那韦的 IC50 为 0.2 μM,抑制 p-糖蛋白介导的沙奎那韦挤出,表明对 p-糖蛋白具有高亲和力。利托那韦的 Ki 值为 13 nM,可有效抑制 ABT-378 的人肝微粒体代谢。虽然利托那韦的效力不如利托那韦 (IC50=0.14 μM),但利托那韦与 ABT-378 组合(比例为 3:1 和 29:1)可抑制 CYP3A(IC50=1.1 和 4.6 μM)。
|
| 动物实验 |
BALB/c mice
60 mg/kg i.p. Animal experiments[8] A cohort of six male Sprague-Dawley rats (in good health, and their individual weights falling within the range of 200–220 g) was used. Prior to commencing the experiment, the rats were housed in a controlled environment with clean cages for a week-long acclimation period. The ambient conditions were maintained at 25 °C and a 12-h light/dark cycle. During this time, the animals enjoyed ad libitum access to food and water. Before the day of dosing, a 12-h fasting period was performed, during which water intake remained unrestricted. Each rat was received an oral administration of a solution containing 30 mg/kg of nirmatrelvir and 10 mg/kg of ritonavir, formulated in 0.5% sodium carboxymethylcellulose. At designated time points, including pre-dose (0 h), 0.33, 0.67, 1, 1.5, 2, 3, 4, 6, 8, 12, 24 and 48 h post-dosing, approximately 0.3 mL of blood was drawn from the tail vein into heparinized centrifuge tubes. After centrifugation of these samples at 8000×g and 25 °C for 10 min, the supernatant was carefully transferred into fresh tubes and stored at −80 °C pending further analysis. Pharmacokinetic parameters of nirmatrelvir and ritonavir in each rat, encompassing area under the concentration-time curve (AUC), time to reach peak plasma concentration (Tmax), maximum plasma concentration (Cmax), elimination half-life (t1/2), apparent clearance (CLz/F), and mean residence time (MRT), were analyzed through non-compartmental statistical models using the Drugs and Statistics (DAS 3.0) software. The data were presented as mean ± standard deviation (SD). Drug repurposing is a promising strategy for identifying new applications for approved drugs. Here, we describe a polymer biomaterial composed of the antiretroviral drug ritonavir derivative (5-methyl-4-oxohexanoic acid ritonavir ester; RD), covalently bound to HPMA copolymer carrier via a pH-sensitive hydrazone bond (P-RD). Apart from being more potent inhibitor of P-glycoprotein in comparison to ritonavir, we found RD to have considerable cytostatic activity in six mice (IC50 ~ 2.3-17.4 μM) and six human (IC50 ~ 4.3-8.7 μM) cancer cell lines, and that RD inhibits the migration and invasiveness of cancer cells in vitro. Importantly, RD inhibits STAT3 phosphorylation in CT26 cells in vitro and in vivo, and expression of the NF-κB p65 subunit, Bcl-2 and Mcl-1 in vitro. RD also dampens chymotrypsin-like and trypsin-like proteasome activity and induces ER stress as documented by induction of PERK phosphorylation and expression of ATF4 and CHOP. P-RD nanomedicine showed powerful antitumor activity in CT26 and B16F10 tumor-bearing mice, which, moreover, synergized with IL-2-based immunotherapy. P-RD proved very promising therapeutic activity also in human FaDu xenografts and negligible toxicity predetermining these nanomedicines as side-effect free nanosystem. The therapeutic potential could be highly increased using the fine-tuned combination with other drugs, i.e. doxorubicin, attached to the same polymer system. Finally, we summarize that described polymer nanomedicines fulfilled all the requirements as potential candidates for deep preclinical investigation.[7] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The absolute bioavailability of ritonavir has not been determined. Following oral administration, peak concentrations are reached after approximately 2 hours and 4 hours (Tmax) after dosing under fasting and non-fasting conditions, respectively. It should be noted that ritonavir capsules and tablets are not considered bioequivalent. Ritonavir is primarily eliminated in the feces. Following oral administration of a single 600mg dose of radiolabeled ritonavir, approximately 11.3 ± 2.8% of the dose was excreted into the urine, of which 3.5 ± 1.8% was unchanged parent drug. The same study found that 86.4 ± 2.9% of the dose was excreted in the feces, of which 33.8 ± 10.8% was unchanged parent drug. The estimated volume of distribution of ritonavir is 0.41 ± 0.25 L/kg. The apparent oral clearance at steady-state is 8.8 ± 3.2 L/h. Renal clearance is minimal and estimated to be <0.1 L/h. Ritonavir and its metabolites are eliminated from the body predominantly in the feces (86% of unchanged drug and metabolites), with minor urinary elimination (11%, mostly metabolites). Absorption of ritonavir is only slightly affected by diet, and this is somewhat dependent on the formulation. The overall absorption of ritonavir from the capsule formulation may increase by 15% when taken with meals. ... There is greater than sixfold variability in drug trough concentrations among patients given 600 mg of ritonavir every 12 hours. The extent of oral absorption is high and is not affected by food. Within the clinical concentration range, ritonavir is approximately 98 to 99% bound to plasma proteins, including albumin and alpha 1-acid glycoprotein. Cerebrospinal fluid (CSF) drug concentrations are low in relation to total plasma concentration. However, parallel decreases in the viral burden have been observed in the plasma, CSF and other tissues. ... About 34% and 3.5% of a 600 mg dose is excreted as unchanged drug in the feces and urine, respectively. The clinically relevant t1/2 beta is about 3 to 5 hours. Because of autoinduction, plasma concentrations generally reach steady state 2 weeks after the start of administration. The pharmacokinetics of ritonavir are relatively linear after multiple doses, with apparent oral clearance averaging 7 to 9 L/hr. Ritonavir is excreted principally in the feces, both as unchanged drug and metabolites. Following oral administration of 600 mg of radiolabeled ritonavir as the oral solution, 86.4% of the dose is excreted in feces (33.8% as unchanged drug) and 11.3% of the dose is excreted in urine (3.5% as unchanged drug). For more Absorption, Distribution and Excretion (Complete) data for RITONAVIR (6 total), please visit the HSDB record page. Metabolism / Metabolites Ritonavir circulates in the plasma predominantly as unchanged drug. Five metabolites have been identified. The isopropylthiazole oxidation metabolite (M-2) is the major metabolite in low plasma concentrations and retains similar antiviral activity to unchanged ritonavir. The cytochrome P450 enzymes CYP3A and CYP2D6 are the enzymes primarily involved in the metabolism of ritonavir. ... Ritonavir is primarily metabolised by cytochrome P450 (CYP) 3A isozymes and, to a lesser extent, by CYP2D6. Four major oxidative metabolites have been identified in humans, but are unlikely to contribute to the antiviral effect. ... Five ritonavir metabolites have been identified in human urine and feces. The isopropylthiazole oxidation metabolite (M2) appears to be the major metabolite. M2 (but not other metabolites) has antiviral activity similar to that of ritonavir; however, only very low concentrations of this metabolite are present in plasma. Other metabolites identified in in vitro studies include a decarbamoylated metabolite (M1) and a product of N-dealkylation at the urea terminus (M11). Biological Half-Life The approximate half-life of ritonavir is 3-5 hours. The clinically relevant t1/2 beta is about 3 to 5 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Some degree of serum aminotransferase elevations occurs in a high proportion of patients taking ritonavir containing antiretroviral regimens. Moderate-to severe elevations in serum aminotransferase levels (>5 times the upper limit of normal) are found in up to 15% of patients treated with full doses of ritonavir and are more common in patients with HIV-HCV coinfection. With low “booster” doses, ritonavir does not appear to increase the frequency or severity of serum enzyme elevations, and those that occur are usually asymptomatic and self-limited, resolving even with continuation of ritonavir. Clinically apparent liver injury from full doses of ritonavir has been reported, but hepatotoxicity from low dose ritonavir has not been clearly linked to acute liver injury. In many situations, the liver injury is difficult to attribute to ritonavir because it is used in combination with higher doses of other protease inhibitors. HIV protease inhibitors have been associated with acute liver injury arising 1 to 8 weeks after onset, with variable patterns of liver enzyme elevation, from hepatocellular to cholestatic. Immunoallergic features (rash, fever, eosinophilia) are uncommon as is autoantibody formation. Ritonavir in combination with saquinavir has also been associated with a rapid onset (1 to 4 days) acute hepatic injury in patients who are taking rifampin and perhaps other agents that affect CYP 450 activity, such as phenobarbital. Finally, initiation of ritonavir based highly active antiretroviral therapy can lead to exacerbation of an underlying chronic hepatitis B or C in coinfected individuals, typically arising 2 to 12 months after starting therapy and associated with a hepatocellular pattern of serum enzyme elevations and increases followed by falls in serum levels of hepatitis B virus (HBV) DNA or hepatitis C virus (HCV) RNA. Ritonavir therapy has not been clearly linked to lactic acidosis and acute fatty liver that is reported in association with several nucleoside analogue reverse transcriptase inhibitors. Likelihood score: C (probable rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Ritonavir is excreted into milk in measurable concentrations and low levels can be found in the blood of some breastfed infants. No adverse reactions in breastfed infants have been reported. Achieving and maintaining viral suppression with antiretroviral therapy decreases breastfeeding transmission risk to less than 1%, but not zero. Individuals with HIV who are on antiretroviral therapy with a sustained undetectable viral load and who choose to breastfeed should be supported in this decision. If a viral load is not suppressed, banked pasteurized donor milk or formula is recommended. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Gynecomastia has been reported among men receiving highly active antiretroviral therapy. Gynecomastia is unilateral initially, but progresses to bilateral in about half of cases. No alterations in serum prolactin were noted and spontaneous resolution usually occurred within one year, even with continuation of the regimen. Some case reports and in vitro studies have suggested that protease inhibitors might cause hyperprolactinemia and galactorrhea in some male patients, although this has been disputed. The relevance of these findings to nursing mothers is not known. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Protein Binding Ritonavir is highly protein-bound in plasma (~98-99%), primarily to albumin and alpha-1 acid glycoprotein over the standard concentration range. Interactions These medications /amiodarone, astemizole, bepridil, bupropion, cisapride, clozapine, dihydroergotamine, encainide, ergotamine, flecainide, meperidine, pimozide, piroxicam, propafenone, propoxyphene, quinidine, rifabutin or terfenadine/ should not be administered concurrently with ritonavir; concurrent administration with ritonavir is likely to produce a large increase in the plasma concentrations of these medications, which may increase the risk of arrhythmias, hematologic abnormalities, seizures, or other potentially serious adverse effects. In one study, concurrent administration /with clarithromycin/ increased the AUC of clarithromycin by 77% and the peak plasma concentration by 31%; dosing does not need to be adjusted in patients with normal renal function; however, for patients with a creatinine clearance of 30 to 60 ml/minute (0.5 to 1 ml/second), the dose of clarithromycin should be reduced by 50%, and for patients with a creatinine clearance of less than 30 ml/minute (0.5 ml/second), the dose of clarithromycin should be reduced by 75%. These medications /clorazepate, diazepam, estazolam, flurazepam, midazolam, triazolam, or zolpidem/ should not be administered concurrently with ritonavir; concurrent administration with ritonavir is likely to produce a large increase in the plasma concentrations of these medications, which may produce extreme sedation and respiratory depression. In one study, concurrent administration /with estrogen-containing oral contraceptive/ decreased the AUC of ethinyl estradiol by 40%; an oral contraceptive with a higher estrogen content or an alternative method of contraception should be considered. For more Interactions (Complete) data for RITONAVIR (14 total), please visit the HSDB record page. |
| 参考文献 |
[1]. Br J Clin Pharmacol . 1997 Aug;44(2):190-4. [2]. J Pharmacol Exp Ther . 1996 Apr;277(1):423-31. [3]. J Hum Virol . 1999 Sep-Oct;2(5):261-9. [4]. Biochem Pharmacol . 1999 May 15;57(10):1147-52. [5]. Drug Metab Dispos . 1999 Aug;27(8):902-8. [6]. Nat Med . 2018 May;24(5):604-609. |
| 其他信息 |
Therapeutic Uses
Ritonavir is indicated in combination with nucleoside analogs or as monotherapy for the treatment of HIV infection or AIDS. /Included in US product labeling/ Lopinavir/ritonavir has demonstrated antiviral activity in the HIV-infected adult. The objective of this study was to investigate a liquid coformulation of lopinavir/ritonavir, in combination with reverse transcriptase inhibitors, in HIV-infected children. One hundred antiretroviral (ARV)-naive and ARV-experienced, nonnucleoside reverse transcriptase inhibitor-naive children between 6 months and 12 years of age participated in this Phase I/II, open label, multicenter trial. Subjects initially received either 230/57.5 mg/sq m or 300/75 mg/sq m lopinavir/ritonavir twice daily; ARV-naive subjects also received stavudine and lamivudine, whereas ARV-experienced subjects also received nevirapine and one or two nucleoside reverse transcriptase inhibitors. Lopinavir/ritonavir pharmacokinetics, safety and efficacy were evaluated. All subjects were escalated to the 300/75 mg/sq m twice daily dose based on results from an interim pharmacokinetic and safety evaluation. The pharmacokinetics of lopinavir did not appear to be dependent on age when dosing was based on body surface area but were decreased on coadministration with nevirapine. Overall 79% of subjects had HIV RNA levels <400 copies/mL at Week 48 (intent-to-treat: missing = failure). Mean increases in absolute and relative (percent) CD4 counts from baseline to Week 48 were observed in both ARV-naive subjects (404 cells/cu mm; 10.3%) and ARV-experienced subjects (284 cells/cu mm; 5.9%). Only one subject prematurely discontinued the study because of a study drug-related adverse event. The liquid coformulation of lopinavir/ritonavir demonstrated durable antiviral activity and was safe and well-tolerated after 48 weeks of treatment in HIV-infected children. Drug Warnings The most frequent adverse effects associated with ritonavir therapy involve the GI tract. In one clinical study in HIV-infected patients, nausea occurred in 25.6%, vomiting in 13.7%, diarrhea in 15.4%, taste perversion in 11.1%, abdominal pain in 6%, local throat irritation in 1.7%, anorexia in 1.7%, and flatulence in 0.9% of patients who received ritonavir monotherapy. In clinical studies in patients with HIV infection who received ritonavir in conjunction with nucleoside antiretroviral therapy or ritonavir in conjunction with saquinavir, nausea occurred in 18.4-46.6%, vomiting in 7.1-23.3%, diarrhea in 22.7-25%, taste perversion in 5-17.2%, anorexia in 4.3-8.6%, abdominal pain in 2.1-8.3%, local throat irritation in 0.9-2.8%, and flatulence in 1.7-3.5% of patients. Constipation, dyspepsia, or fecal incontinence occurred in 0.2-3.4, 0.7-5.9, or 2.8%, respectively, of patients receiving ritonavir with other antiretroviral agents; these effects were not reported in patients receiving ritonavir monotherapy. Many adverse GI effects reported with ritonavir are transient; vomiting persists for an average of 1 week, nausea for 2-3 weeks, and diarrhea for 5 weeks. Adverse GI effects reported in less than 2% of patients receiving ritonavir alone or in conjunction with other antiretroviral agents include abnormal stools, bloody diarrhea, cheilitis, cholangitis, colitis, dry mouth, dysphagia, enlarged abdomen, eructation, esophageal ulcer, esophagitis, gastritis, gastroenteritis, GI disorder, GI hemorrhage, gingivitis,ileus, melena, mouth ulcer, pseudomembranous colitis, rectal disorder, rectal hemorrhage, sialadenitis, stomatitis, taste loss, tenesmus, thirst, tongue edema, and ulcerative colitis. Peripheral paresthesia occurred in 6% and paresthesia or circumoral paresthesia occurred in 2.6-3.4% of patients with HIV infection receiving ritonavir monotherapy in one clinical study (study 245). In clinical studies in patients receiving ritonavir in conjunction with nucleoside antiretroviral therapy (studies 245 and 247) or in conjunction with saquinavir (study 462), peripheral paresthesia was reported in 55.7%, paresthesia in 2.1-5.2%, and circumoral paresthesia in 5.2-6.7% of patients. Asthenia occurred in 10.3% of patients receiving ritonavir monotherapy and in 15.3-28.4% of patients receiving ritonavir with other antiretroviral agents. Many of these adverse effects are transient; peripheral paresthesia persists for an average of 34 weeks and circumoral paresthesia and asthenia persist for 35 weeks. Dizziness, insomnia, or somnolence have been reported in 2.6% of patients receiving ritonavir monotherapy and in 3.9-8.5, 2-3.4, or 2.4-2.6%, respectively, of patients receiving ritonavir with other antiretroviral agents. Headache, depression, or abnormal thinking were reported in 4.3-7.8, 1.7-7.1, or 0.7-2.6%, respectively, of patients receiving ritonavir in conjunction with other antiretroviral agents. Anxiety or confusion were reported in up to 2.1% of patients receiving ritonavir with other antiretroviral agents. For more Drug Warnings (Complete) data for RITONAVIR (34 total), please visit the HSDB record page. Pharmacodynamics Ritonavir is a protease inhibitor with activity against Human Immunodeficiency Virus Type 1 (HIV-1). Protease inhibitors block the part of HIV called protease. HIV-1 protease is an enzyme required for the proteolytic cleavage of the viral polyprotein precursors into the individual functional proteins found in infectious HIV-1. Ritonavir binds to the protease active site and inhibits the activity of the enzyme. This inhibition prevents cleavage of the viral polyproteins resulting in the formation of immature non-infectious viral particles. Protease inhibitors are almost always used in combination with at least two other anti-HIV drugs. Modern protease inhibitors require the use of low-dose ritonavir to boost pharmacokinetic exposure through inhibition of metabolism via the cytochrome P450 3A4 enzyme pathway. |
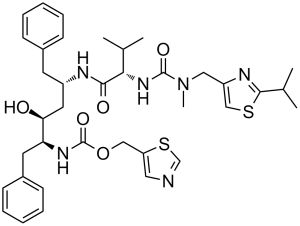
| 分子式 |
C37H48N6O5S2
|
|---|---|
| 分子量 |
720.94
|
| 精确质量 |
720.312
|
| 元素分析 |
C, 61.64; H, 6.71; N, 11.66; O, 11.10; S, 8.90
|
| CAS号 |
155213-67-5
|
| 相关CAS号 |
Ritonavir-d6;1616968-73-0;rel-Ritonavir-d6;1217720-20-1;Ritonavir metabolite;176655-55-3;Ritonavir-13C,d3
|
| PubChem CID |
392622
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
947.0±65.0 °C at 760 mmHg
|
| 熔点 |
120-122°C
|
| 闪点 |
526.6±34.3 °C
|
| 蒸汽压 |
0.0±0.3 mmHg at 25°C
|
| 折射率 |
1.600
|
| LogP |
5.28
|
| tPSA |
202.26
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
18
|
| 重原子数目 |
50
|
| 分子复杂度/Complexity |
1040
|
| 定义原子立体中心数目 |
4
|
| SMILES |
S1C([H])=C(C([H])([H])N(C([H])([H])[H])C(N([H])[C@]([H])(C(N([H])[C@@]([H])(C([H])([H])C2C([H])=C([H])C([H])=C([H])C=2[H])C([H])([H])[C@@]([H])([C@]([H])(C([H])([H])C2C([H])=C([H])C([H])=C([H])C=2[H])N([H])C(=O)OC([H])([H])C2=C([H])N=C([H])S2)O[H])=O)C([H])(C([H])([H])[H])C([H])([H])[H])=O)N=C1C([H])(C([H])([H])[H])C([H])([H])[H]
|
| InChi Key |
NCDNCNXCDXHOMX-XGKFQTDJSA-N
|
| InChi Code |
InChI=1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1
|
| 化学名 |
1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[[(2S)-3-methyl-2-[[methyl-[(2-propan-2-yl-1,3-thiazol-4-yl)methyl]carbamoyl]amino]butanoyl]amino]-1,6-diphenylhexan-2-yl]carbamate
|
| 别名 |
ABT-538; A 84538; Norvir; ABT538; Norvir; ABT-538; A-84538; Abbott 84538; ABBOTT-84538; Empetus; A-84538; Norvir Sec; 538, ABT; Ritonavir; ABT 538;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.5 mg/mL (3.47 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (3.47 mM) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: 2.5 mg/mL (3.47 mM) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 配方 4 中的溶解度: 2.5 mg/mL (3.47 mM) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 5 中的溶解度: 0.5 mg/mL (0.69 mM) in 1% DMSO 99% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 6 中的溶解度: 30% PEG400+0.5% Tween80+5% propylene glycol: 30 mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.3871 mL | 6.9354 mL | 13.8708 mL | |
| 5 mM | 0.2774 mL | 1.3871 mL | 2.7742 mL | |
| 10 mM | 0.1387 mL | 0.6935 mL | 1.3871 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Bone, Immunologic, and Virologic Effects of a Antiretroviral Regimen
CTID: NCT01400412
Phase: Phase 2 Status: Completed
Date: 2024-10-15
|
|---|
|